La vascularite cérébrale – adulte ou pédiatrique – est une tempête silencieuse dans le cerveau. Il s’agit d’une maladie auto-immune inflammatoire des vaisseaux sanguins, qui provoque des accidents vasculaires cérébraux et altère les fonctions cognitives. Les traitements standards sont basés sur des immunosuppresseurs puissants, qui diminuent l’inflammation en échange de symptômes comme la fatigue, la prédisposition aux infections et une dépendance à long terme. La thérapie par cellules souches de la vascularite du système nerveux central (SNC) offre des résultats prometteurs lorsqu’elle est utilisée en conjonction avec le traitement conventionnel. Elle vise non seulement à limiter la maladie, mais aussi à favoriser la régénération du cerveau, accélérant ainsi la guérison.
Cet article traite des points suivants:
- Définition de la vascularite cérébrale,
- Vascularite cérébrale : causes,
- Vascularite cérébrale : symptômes,
- Vascularite cérébrale : traitement, et pourquoi cette approche offre un nouvel espoir aux personnes vivant avec cette maladie difficile.
Les maladies auto-immunes et le cerveau
Les maladies auto-immunes surviennent lorsque les cellules immunitaires de l’organisme – normalement chargées de détruire les envahisseurs étrangers tels que les virus ou les bactéries – ne fonctionnent pas correctement.
En conséquence, les leucocytes commencent à attaquer les propres cellules de l’organisme, les prenant pour des menaces.
Dans les affections cérébrales auto-immunes, le système immunitaire cible par erreur les tissus cérébraux. L’inflammation chronique érode les parois des vaisseaux, altère l’intégrité de la barrière hémato-encéphalique et favorise la neurodégénérescence.
Dans le cas de la vascularite du système nerveux central, ces effets peuvent être particulièrement graves. Les patients peuvent souffrir de maux de tête persistants, de confusion, de pertes de mémoire, de crises d’épilepsie, voire d’accidents vasculaires cérébraux. Au fur et à mesure que la maladie s’aggrave, elle peut entraver de manière significative le fonctionnement quotidien, réduire l’indépendance et affecter profondément la qualité de vie. Un diagnostic précoce et un traitement efficace sont essentiels pour prévenir les dommages neurologiques à long terme.
Définition
La vascularite cérébrale – adulte ou pédiatrique – est une inflammation des vaisseaux sanguins du cerveau. Elle perturbe la circulation sanguine, endommage les tissus environnants et peut entraîner des symptômes neurologiques allant de légères modifications cognitives à de graves déficiences. Cette inflammation peut être due à des infections, à des maladies auto-immunes comme le lupus, ou peut survenir sans cause précise – appelée vascularite cérébrale primaire idiopathique.
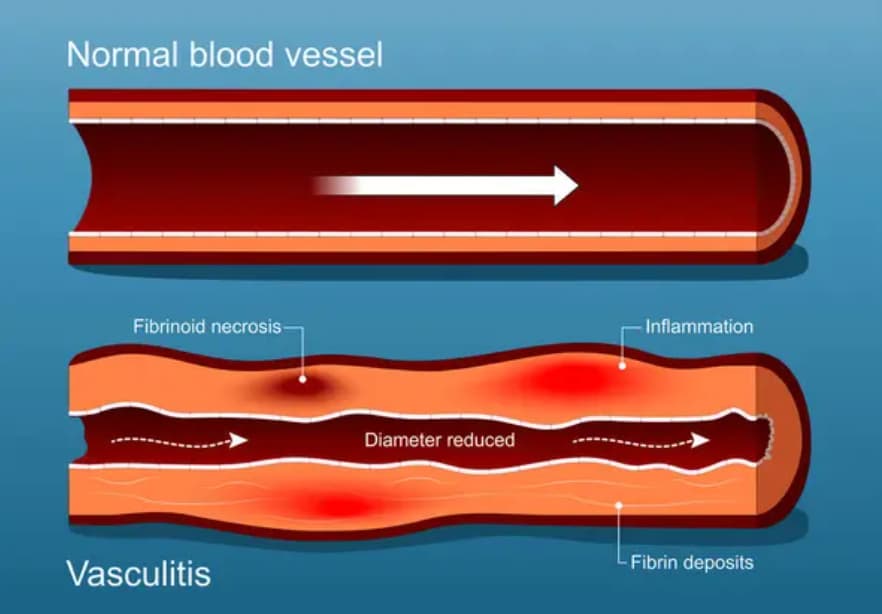
Vascularite cérébrale : causes et facteurs de risque
Les causes de la vascularite cérébrale varient et peuvent impliquer l’interaction de facteurs immunitaires, infectieux et génétiques.
- Les infections qui peuvent être déclenchantes sont le virus de la varicelle et du zona (VZV), le virus de l’immunodéficience humaine (VIH), le virus de l’hépatite C et des bactéries telles que Mycoplasma, Rickettsia et Treponema.
- Les maladies auto-immunes systémiques et les maladies du tissu conjonctif telles que le lupus érythémateux disséminé (LED), la maladie de Behçet, le syndrome de Sjögren et le syndrome de Churg et Strauss peuvent y contribuer ou même y ressembler.
- Le sexe : Elles sont généralement plus fréquentes chez les hommes, mais l’artérite à cellules géantes est plus fréquente chez les femmes.
Vascularite cérébrale : symptômes et diagnostic
Le diagnostic de cette maladie est complexe, car les symptômes peuvent imiter d’autres troubles neurologiques. La relation entre vascularite cérébrale et symptôme reste parfois subtile au début ou menace le pronostic vital. Les manifestations les plus courantes sont les suivantes :
- Changements de la vision
- Maux de tête persistants
- Troubles de la mémoire
- Vascularite cérébrale et symptôme moteur : faiblesse musculaire
- Crises d’épilepsie ou épisodes ressemblant à des accidents vasculaires cérébraux
Un diagnostic et une intervention rapides sont essentiels pour éviter des dommages irréversibles. Les procédures de diagnostic comprennent l’imagerie cérébrale (IRM, angiographie), l’analyse du liquide céphalorachidien et parfois une biopsie du tissu cérébral. Les lignes directrices relatives à la vascularite cérébrale, telles que celles figurant dans les rubriques Vascularite cérébrale d’eMedicine ou Traitement de la vascularite du SNC d’UpToDate, aident les médecins à établir le diagnostic différentiel.
Vascularite cérébrale : traitement traditionnel
Les traitements actuels de la vascularite cérébrale font généralement appel à des médicaments immunosuppresseurs pour contrôler les symptômes et prévenir la progression de la maladie.
Médicaments et thérapies
- Corticostéroïdes pour réduire l’inflammation ;
- Médicaments immunosuppresseurs tels que le cyclophosphamide ou l’azathioprine ;
- Plasmaphérèse dans certains cas d’auto-immunité.
Limites des traitements conventionnels
Si ces traitements permettent de gérer les poussées, ils n’inversent pas les lésions tissulaires. Une utilisation prolongée peut entraîner des effets secondaires graves, notamment
- un risque accru d’infection
- un déséquilibre hormonal
- des lésions organiques.
C’est là que les approches régénératives, telles que la thérapie par cellules souches mésenchymateuses (CSM), entrent en jeu.
Thérapie par cellules souches pour la vascularite cérébrale
Les avancées en médecine régénérative ont permis d’explorer le lien entre cellule souche et traitement de nombreuses pathologies auparavant incurables. La thérapie par cellules souches mésenchymateuses est une forme de médecine régénérative qui utilise les mécanismes naturels de réparation de l’organisme pour gérer les maladies chroniques. La prise en charge de cette maladie par les cellules souches ne consiste pas à remplacer les cellules endommagées, mais à aider l’organisme à se réparer lui-même.
Mécanismes d’action
Les cellules souches sont des cellules spéciales qui peuvent trouver des zones endommagées ou enflammées dans le corps. Lorsqu’elles atteignent ces zones, les cellules libèrent des substances utiles, telles que des facteurs de croissance et des protéines, qui favorisent la cicatrisation, réduisent l’inflammation et aident à réparer les tissus environnants. Ces signaux soutiennent les processus de régénération de l’organisme et aident à protéger les tissus environnants contre des dommages supplémentaires. Dans ce contexte particulier, les CSM aident à protéger les neurones et les parois des vaisseaux sanguins contre d’autres lésions en réduisant le stress oxydatif.
Pourquoi Swiss Medica n’utilise-t-elle que des CSM adultes ?
Swiss Medica n’utilise que des cellules souches mésenchymateuses (CSMs) adultes et évite les cellules souches embryonnaires ou pluripotentes induites (iPSC) pour des raisons éthiques et en raison des risques de formation de tumeurs.
Types de cellules souches utilisées
Nos produits à base de cellules souches proviennent de différentes sources, chacune offrant des avantages thérapeutiques uniques, adaptés à des besoins spécifiques :
| Source | Type de cellule ou produit | Description et avantages |
| Cordon ombilical ou tissu placentaire | CSM de donneur | Il s’agit de cellules d’origine éthique prélevées après la naissance. Elles sont riches en potentiel régénératif et ont un taux de prolifération élevé. Elles conviennent parfaitement à la gestion de l’inflammation systémique et à la régulation du système immunitaire. |
| Moelle osseuse ou tissu adipeux | CSM autologues | Il s’agit des cellules souches propres au patient, généralement extraites de la moelle osseuse ou du tissu adipeux. Elles sont particulièrement utiles pour ceux qui préfèrent ne pas utiliser de cellules de donneurs. |
| Dérivés acellulaires | Exosomes | Il ne s’agit pas de cellules entières mais de molécules bioactives libérées par les CSM. Ils favorisent la cicatrisation, réduisent l’inflammation et sont utilisés dans les soins de suivi. |
Le système immunitaire ne reconnaît pas ces produits biomédicaux comme étrangers, ce qui rend le rejet extrêmement improbable. Chaque lot fait l’objet de tests approfondis de stérilité, de stabilité génétique et d’efficacité.
L’approche des cellules souches vous intéresse ? Pour en savoir plus, consultez notre article sur les cellules souches utilisées en thérapie.
En savoir plusTraitements à base de cellules souches pour la vascularite cérébrale : avantages et risques
Les experts cliniques étudient le traitement des symptômes de la vascularite cérébrale à l’aide d’approches innovantes basées sur les cellules souches. Bien qu’il n’y ait pas de remède permanent, les thérapies régénératives offrent des améliorations substantielles de la fonction et de l’endurance. Principaux avantages :
- Réduire l’inflammation à la source.
- Stimuler la réparation des tissus vasculaires et neuronaux.
Les patients doivent éviter la thérapie s’ils répondent aux critères suivants :
- Cancer actif – en raison des interactions potentielles avec les processus pathologiques en cours.
- Grossesse ou allaitement – pour garantir la sécurité de la mère et de l’enfant.
- Infections non contrôlées – le système immunitaire doit être stable pour obtenir des résultats optimaux.
Les consultations comprennent un examen des antécédents médicaux et un dépistage pour s’assurer de l’admissibilité et de la sécurité.
Obtenez une consultation en ligne gratuite
Réservez votre consultation gratuite en ligne avec notre spécialiste en médecine régénérative pour savoir si le traitement par cellules souches de la vascularite cérébrale vous convient.

Medical Advisor, Swiss Medica doctor
Admissibilité des patients et considérations
Les patients éligibles à la thérapie par cellules souches pour la vascularite cérébrale présentent plusieurs caractéristiques communes.
Vous pouvez être considéré comme un candidat à la thérapie par cellules souches si
- Votre maladie reste active malgré un traitement médicamenteux continu ;
- Vous êtes à la recherche d’options thérapeutiques autres que les médicaments immunosuppresseurs ;
- Vous êtes actuellement dans un état de santé stable, sans infection active ni cancer.
Pour beaucoup, l’objectif n’est pas seulement de contrôler les symptômes, mais aussi la guérison à long terme de la vascularite cérébrale, c’est-à-dire l’amélioration des fonctions cognitives et neurologiques et une plus grande stabilité de l’état de santé général.
Bien que la thérapie par cellules souches ne garantisse pas la guérison, elle peut offrir des améliorations significatives des symptômes et de la qualité de vie, en particulier lorsque les traitements conventionnels ne suffisent plus.
Processus de traitement de la vascularite cérébrale par cellules souches
Si vous envisagez un traitement par cellules souches, il est essentiel de comprendre l’ensemble du processus de traitement. Chez Swiss Medica, nous accordons la priorité à la sécurité, à la transparence et aux soins individualisés à chaque étape. Voici ce à quoi vous pouvez généralement vous attendre :
Évaluation médicale
Le processus commence par un examen détaillé des antécédents médicaux du patient, de ses symptômes, de ses traitements antérieurs et de l’imagerie diagnostique. Nos spécialistes évaluent la nature et la gravité de la vascularite cérébrale, effectuent des tests de laboratoire pour éliminer les contre-indications et déterminent si le traitement par cellules souches est approprié et sûr.

Nous disposons de notre propre laboratoire à la clinique Swiss Medica. Cela nous permet de contrôler la qualité des produits biomédicaux utilisés dans la thérapie à chaque étape de la procédure. Protocole personnalisé
Sur la base des résultats de l’évaluation, nos médecins élaborent un plan de traitement sur mesure. Il s’agit notamment de sélectionner le type de cellules approprié, soit des CSM provenant d’un donneur et issues d’un tissu ombilical ou placentaire, soit des CSM autologues issues de la moelle osseuse ou du tissu adipeux du patient. Le protocole peut également intégrer des éléments de soutien tels que des exosomes pour améliorer la récupération neurologique. D’autres thérapies de support, telles que les perfusions de nutriments, la physiothérapie ou les traitements antioxydants, peuvent être incluses pour améliorer la fonction cellulaire et les résultats globaux.
Administration
La méthode d’administration des cellules dépend des besoins spécifiques du patient et de la gravité de son état. La perfusion intraveineuse (IV) est couramment utilisée pour s’assurer que les cellules souches circulent dans la circulation sanguine, réduisant ainsi l’inflammation systémique.
Pour des effets neurologiques plus ciblés, l’injection intrathécale – l’administration de cellules souches directement dans le canal rachidien – peut être recommandée. Cela permet aux cellules de contourner la barrière hémato-encéphalique et d’atteindre plus efficacement les régions cérébrales enflammées. Le choix de la voie d’administration se fait en fonction de la sécurité, des objectifs cliniques et des facteurs de santé individuels.
Surveillance et rétablissement
Après l’injection de cellules souches, les patients restent sous surveillance étroite afin de contrôler leur réaction et d’assurer leur confort et leur sécurité immédiats. Les signes vitaux et l’état neurologique sont vérifiés régulièrement. Notre équipe médicale travaille 24 heures sur 24 pour gérer les effets secondaires à court terme, tels que la fièvre ou les maux de tête. Les patients ne quittent pas la clinique immédiatement après le traitement ; au contraire, après l’administration de cellules souches pour la vascularite cérébrale, une période de récupération est nécessaire. Au cours de cette période, les patients reçoivent des soins personnalisés et des recommandations de suivi. Après la sortie de l’hôpital, nos spécialistes continuent à surveiller le traitement à distance et à fournir un soutien supplémentaire si nécessaire.
Nous proposons un protocole de thérapie à domicile de 5 semaines avec des exosomes pour les patients se remettant d’une maladie du SNC, dont la vascularite cérébrale.
Coût et accessibilité
Par rapport aux pays occidentaux, les centres de cellules souches d’Europe de l’Est et d’Asie proposent des prix plus accessibles. Les traitements de Swiss Medica varient de 7 000 € à 31 000 €* en fonction de la gravité de l’affection, du protocole et de la durée. Les diagnostics complets et le suivi sont inclus.
*Les prix mentionnés sont indicatifs et susceptibles d’être modifiés en fonction de facteurs individuels, notamment la gravité de l’affection et le nombre de cellules souches nécessaires. Les prix sont valables à partir de janvier 2025.
Comment choisir la bonne clinique
Voici une liste de certains des critères que nous recommandons pour l’évaluation du centre de votre choix :
- Recherchez une transparence clinique et la disposition de laboratoires sur place ;
- Vérifiez l’utilisation de protocoles de cellules souches mésenchymateuses certifiés ;
- Envisagez une procédure de suivi, en particulier pour les cas complexes des atteintes du SNC.
Swiss Medica, basé à Belgrade, en Serbie, répond à tous ces critères, avec plus de 10 000 patients traités et des taux de réussite allant jusqu’à 80 %. Son expertise en matière de thérapie par cellules souches garantit des soins personnalisés conformes aux normes de sécurité internationales. La Serbie est le meilleur pays pour le traitement par cellules souches, car il suffit de deux heures de vol depuis n’importe quel pays européen et les prix des thérapies sont relativement abordables.
Vous vous demandez combien coûte un traitement à base de cellules souches et s’il en vaut la peine ? Swiss Medica offre plus qu’une simple thérapie régénérative de pointe – nous offrons une expérience de confiance, centrée sur le patient, et basée sur plus d’une décennie d’expertise. Voici ce qui nous distingue :
- Des produits cellulaires d’origine éthique : Nous n’utilisons que des cellules souches mésenchymateuses adultes. Nous n’utilisons jamais de cellules embryonnaires ou controversées. Cela garantit la sécurité et la conformité aux normes internationales.
- Une clinique de pointe : Notre clinique principale à Belgrade dispose de son propre laboratoire, d’une cryobanque et d’une assistance médicale disponible 24 heures sur 24 et 7 jours sur 7 dans cinq bâtiments modernes.
- Protocoles personnalisés : Chaque patient reçoit un plan personnalisé utilisant plus de 31 produits cellulaires et acellulaires certifiés, y compris des exosomes pour la thérapie à domicile.
- Modèle de soins complets : Nous soutenons chaque phase de votre rétablissement par des soins médicaux, une réadaptation et un soutien émotionnel.
Par ailleurs, vous pouvez explorer davantage sur le tourisme et cellules souches en consultant ce lien.
La nouvelle clinique de Swiss Medica, ouverte en 2024, présente une architecture moderne, une atmosphère chaleureuse, une technologie de pointe et une équipe compétente de prestataires de soins de santé.
Études cliniques et preuves scientifiques
Des preuves scientifiques de plus en plus nombreuses suggèrent que les cellules stromales mésenchymateuses (CSM) pourraient aider à traiter des affections cérébrales inflammatoires telles que la vascularite cérébrale.
Une étude récente a montré que les CSM contribuaient à réduire la neuroinflammation en libérant des facteurs immunomodulateurs et en favorisant la stabilité de la barrière hémato-encéphalique – une préoccupation majeure dans la vascularite cérébrale.
De même, une revue a mis en évidence la capacité des CSM à supprimer les cellules immunitaires hyperactives tout en favorisant la réparation des tissus. Ces qualités rendent la thérapie par les CSM particulièrement précieuse dans les maladies auto-immunes, où les traitements standard peuvent supprimer le système immunitaire mais n’inversons pas les dommages.
Bien que les recherches spécifiquement liées à la vascularite cérébrale restent limitées en raison de sa rareté, les études sur les maladies cérébrales auto-immunes connexes soutiennent fortement l’utilisation des CSM en tant que voie de traitement prometteuse, sûre et éthique.
Curieux de savoir comment nous traitons d’autres maladies auto-immunes ou neurologiques ? Consultez nos sections sur la sclérose en plaques.
Explorer les sectionsOrientations futures du diagnostic et du traitement de la vascularite cérébrale
Vivre avec une vascularite cérébrale est accablant. Aujourd’hui, les patients ont accès à plus qu’un simple contrôle des symptômes.
Les rapports préliminaires et les commentaires des patients sur les maladies apparentées font état d’améliorations notables, telles que :
- Un meilleur contrôle des mouvements du corps et de la coordination ;
- Une réduction de l’intensité et de la fréquence des maux de tête ou des épisodes neurologiques ;
- Une amélioration de la clarté cognitive et de la concentration ;
- Une stabilité émotionnelle et une énergie accrues ;
- Une amélioration de l’ensemble des symptômes et de l’indépendance dans la vie quotidienne.
Les CSM peuvent améliorer les symptômes de nombreuses maladies chroniques, y compris la vascularite cérébrale. Ces caractéristiques font de cette approche une solution prometteuse pour les patients.
Contactez-nous
Si vous ou l’un de vos proches envisagez d’autres solutions, pensez à prendre rendez-vous pour une consultation gratuite avec notre équipe de spécialistes.
Pour les curieux, consultez également cet article sur l’injection de cellules souches au visage et prix.

Medical Advisor, Swiss Medica doctor
Foire Aux Questions
1. Peut-on guérir la vascularite cérébrale ?
Bien qu’il n’existe pas encore de traitement universel de la vascularite cérébrale, la thérapie par cellules souches offre l’une des voies les plus prometteuses vers un soulagement à long terme et une récupération fonctionnelle. Elle aide à gérer les symptômes, à réduire l’inflammation et à promouvoir la réparation du tissu cérébral – des étapes essentielles pour une amélioration significative.
2. Quelle est la durée des effets ?
Les CSM restent actives pendant environ 6 mois, mais les effets bénéfiques peuvent durer plus longtemps.
3. L’intervention est-elle douloureuse ?
La plupart des procédures sont indolores et n’impliquent que des injections intraveineuses ou rachidiennes.
4. Cette thérapie est-elle soutenue par le site eMedicine sur la vascularite cérébrale ?
Alors que les recherches sur la gestion de la vascularite du SNC sont toujours en cours, les approches régénératives sont de plus en plus fréquemment mentionnées par des plateformes faisant autorité dans le cadre des soins de soutien.
Liste des Références:
Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020 Jul;77(14):2771-2794. doi: 10.1007/s00018-020-03454-6. Epub 2020 Jan 21. PMID: 31965214; PMCID: PMC7223321.
Sheikhi K, Ghaderi S, Firouzi H, Rahimibarghani S, Shabani E, Afkhami H, Yarahmadi A. Recent advances in mesenchymal stem cell therapy for multiple sclerosis: clinical applications and challenges. Front Cell Dev Biol. 2025 Feb 3;13:1517369. doi: 10.3389/fcell.2025.1517369. PMID: 39963155; PMCID: PMC11830822.
Rash, B.G., Ramdas, K.N., Agafonova, N. et al. Allogeneic mesenchymal stem cell therapy with laromestrocel in mild Alzheimer’s disease: a randomized controlled phase 2a trial. Nat Med 31, 1257–1266 (2025). https://doi.org/10.1038/s41591-025-03559-0
Quan J, Liu Q, Li P, Yang Z, Zhang Y, Zhao F, Zhu G. Mesenchymal stem cell exosome therapy: current research status in the treatment of neurodegenerative diseases and the possibility of reversing normal brain aging. Stem Cell Res Ther. 2025 Feb 21;16(1):76. doi: 10.1186/s13287-025-04160-5. PMID: 39985030; PMCID: PMC11846194.
Godasi R, Pang G, Chauhan S, et al. Primary Central Nervous System Vasculitis. [Updated 2023 Jun 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482476/
Yuan MQ, Song L, Wang ZR, Zhang ZY, Shi M, He J, Mo Q, Zheng N, Yao WQ, Zhang Y, Dong T, Li Y, Zhang C, Song J, Huang L, Xu Z, Yuan X, Fu JL, Zhen C, Cai J, Dong J, Zhang J, Xie WF, Li Y, Zhang B, Shi L, Wang FS. Long-term outcomes of mesenchymal stem cell therapy in severe COVID-19 patients: 3-year follow-up of a randomized, double-blind, placebo-controlled trial. Stem Cell Res Ther. 2025 Feb 25;16(1):94. doi: 10.1186/s13287-025-04148-1. PMID: 40001244; PMCID: PMC11863646.
Salvarani C, Brown RD Jr, Christianson TJH, Huston J 3rd, Giannini C, Hunder GG. Long-term remission, relapses and maintenance therapy in adult primary central nervous system vasculitis: A single-center 35-year experience. Autoimmun Rev. 2020 Apr;19(4):102497. doi: 10.1016/j.autrev.2020.102497. Epub 2020 Feb 13. PMID: 32062032.
Constantin Hecker, Tobias Welponer, Manfred Herold, Eugen Trinka, Erasmia Broussalis, Monika Killer-Oberpfalzer, Update on treatment strategies for vasculitis affecting the central nervous system, Drug Discovery Today, Volume 27, Issue 4, 2022, Pages 1142-1155. ISSN 1359-6446, https://doi.org/10.1016/j.drudis.2021.11.020.
Liao, Lianming. Mesenchymal stem cell and hematopoietic stem cell transplantation for vasculitis. Vascular Investigation and Therapy 3(3):p 88-93, Jul–Sep 2020. | DOI: 10.4103/VIT.VIT_20_20
Wang, Y., Yi, H. & Song, Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther 12, 545 (2021). https://doi.org/10.1186/s13287-021-02609-x
MD, Pediatrician, Regenerative Medicine Specialist












