Regenerative medicine is moving fast. Almost every year, new treatments based on stem cells appear in the news. One of the recent highlights in stem cell research is the discovery of Muse cells. In fact, these cells were first discovered back in 2010, but they have come into the spotlight now thanks to promising clinical trial results.
But what is Muse cell therapy and how do these cells work? Primarily, they have several important advantages for regenerative medicine: they are naturally present in the human body, can survive in harsh environments like low oxygen, and, when combined with mesenchymal stem cells (MSCs), can promote healing processes in the body. Muse cells are being studied for a wide range of conditions—from neurodegenerative diseases like Parkinson’s and ALS to autoimmune disorders and heart attacks.
Getting to Know Muse Cells
Muse cells—short for Multilineage-differentiating Stress Enduring cells—are a type of stem cell within the larger family of MSCs. While MSCs are multipotent and mainly support the formation of mesodermal tissues such as bone, cartilage, fat, and muscle, Muse cells are pluripotent. It means that they have a broader potential and can differentiate into nerve cells, skin, liver, and muscle.
Unlike embryonic stem cells or induced pluripotent stem cells (iPSCs), Muse cells occur naturally in the adult human body, remain stable, and do not form tumors. Your bone marrow, for example, constantly releases small numbers of these cells into your bloodstream to detect damage.
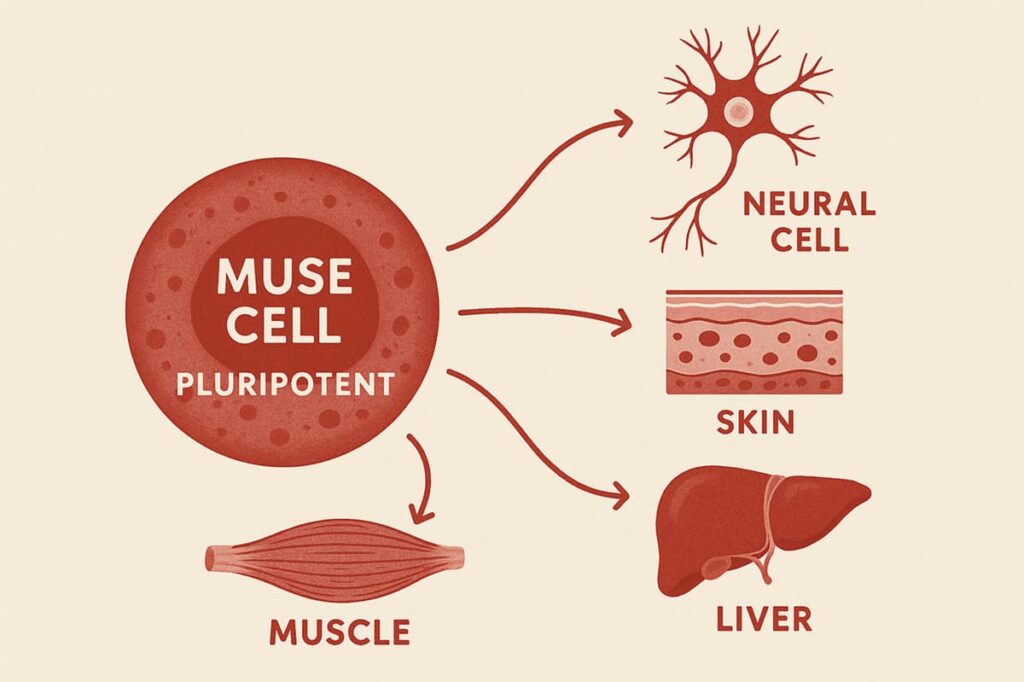
Researchers have identified Muse cells in:
- Bone marrow
- Fat tissue
- Connective tissues, like skin dermis
- Circulating blood
- Birth tissues like the umbilical cord
Although they sound powerful, Muse cells are rare. They make up only about 1% of MSCs, so special laboratory methods are required to collect enough for therapy.
Their rarity distinguishes them as next-generation stem cells: despite being a minority, they seem to play an outsized role in natural repair. As researchers report, Muse cells show great potential for supporting tissue regeneration in chronic diseases and complex conditions, particularly when used in combination with regular MSCs.
Want to see the bigger picture? Discover the different stem cell types used in regenerative medicine and how they work in our dedicated article.
Read moreThe Science Behind Muse Cells
Let’s explore what Muse stem cells can—and cannot—do, based on current scientific evidence.
Stress Resistance That Sets Them Apart
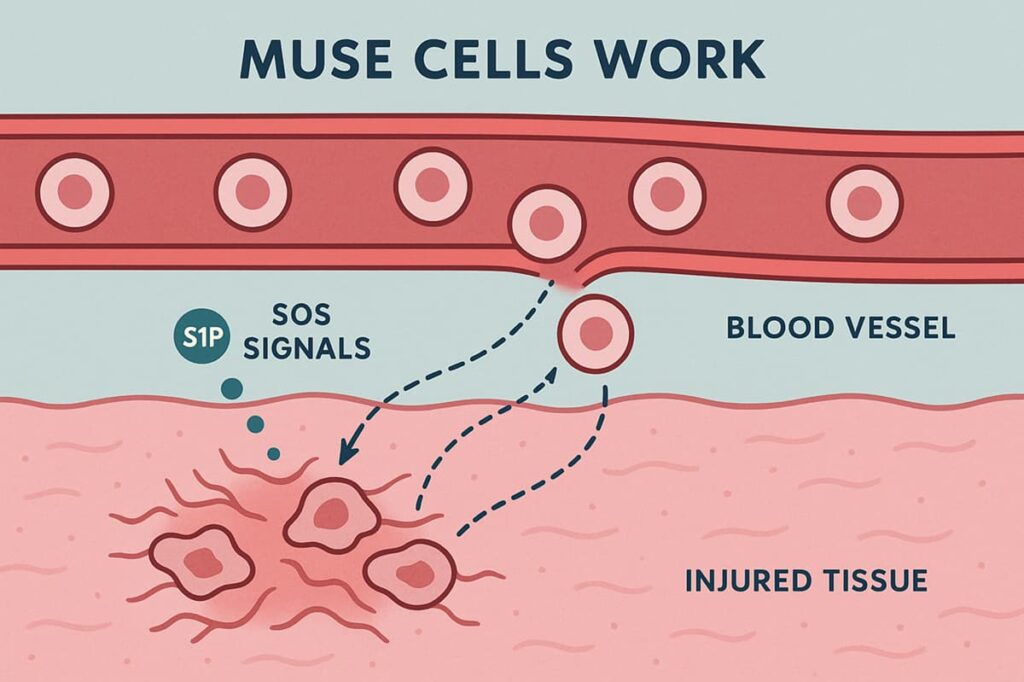
Like regular MSCs, Muse cells actively migrate toward injured tissues, responding to chemical “SOS signals” released by damaged cells. A key signal is the molecule sphingosine-1-phosphate (S1P), which creates a gradient that guides Muse cells out of the bloodstream and directly to the site of injury. This targeted migration enables them to reach damaged areas quickly and initiate repair with high precision.
Once at the injury site, Muse cells demonstrate a high ability to survive in environments that are typically hostile to transplanted cells. Injury sites are often deprived of oxygen, saturated with inflammatory molecules, and rich in free radicals—all factors that threaten cell survival.
The ability of Muse cells to survive in complex conditions enhances the effectiveness of regular MSCs. This resilience allows MSC-based therapy to better withstand hostile environments, reduce inflammation, and promote tissue repair, leading to stronger therapeutic outcomes.
Pluripotency Without the Tumor Risk
Pluripotent cells can develop into many different tissue types, but this ability is sometimes linked to concerns about tumor formation, especially with embryonic stem cells. Muse stem cells are different. They are a naturally occurring subpopulation within MSCs—a cell type well established for its safety and lack of tumor-forming behavior. Although Muse cells possess pluripotent-like potential, early clinical research shows that they retain the same safety profile as MSCs and do not form tumors.
Initial studies suggest that Muse cells may be inherently safer, partly because of their low telomerase activity and natural regulatory mechanisms. However, research is still in progress, and larger, long-term studies are needed to fully confirm their safety profile.
For patients, this means that Muse stem cell treatments, when combined with regular MSCs, may provide the regenerative power of embryonic-like cells while avoiding the cancer risk.
To help you understand the proven safety of MSCs, we reviewed the available studies and explained the mechanisms behind their safety profile. Read more in our detailed article.
Read moreHow Muse Cells Actually Work in Your Body
Muse cell innovation is characterized by their similar abilities to regular MSCs, shown through three main ways they work in damaged tissues.
Once introduced into a patient’s body, Muse cells circulate and actively seek out areas of injury. They are “listening” for distress signals in the form of S1P, a molecule abundant in injured tissue. It enables them to detect injured areas more accurately and respond faster.
Turning Into What You Need: The Differentiation Process
Muse cells, like regular MSCs, quickly adapt to their environment, differentiating into the needed cell type within just a few days. At a muscle injury, they may form new muscle fibers; in a spinal cord lesion, they may differentiate into neural cells; and at a skin wound, they may generate fresh skin cells—right at the site of damage.
This transformation happens because signals from damaged cells guide the Muse cells, activating the right parts of their DNA. As a result, Muse cells can accurately replace many different types of injured cells and even form new blood vessels, which are essential for proper healing.
It doesn’t mean the cells rebuild entire organs or tissues on their own—similar to MSCs, they mainly boost the body’s natural healing ability.
To understand this better, start with the basics: learn how MSCs work. This foundation will make it clearer how Muse cells may support your body’s natural recovery.
Read moreCalming Inflammation: The Anti-Inflammatory Side
Repairing structural damage is only half the battle—many chronic diseases are fueled by inflammation and overactive immune responses. Muse cells, like other MSC populations, play an important role here.
Upon reaching the damaged site, these cells exit the blood vessels and start releasing growth factors:
- Chemokines that promote cell migration,
- Adhesion molecules that regulate interactions at the cellular level.
- Anti-inflammatory cytokines to calm inflammation.
This process creates a supportive microenvironment that helps damaged tissue recover. Muse stem cells, like other MSCs, help to clean up debris and modulate inflammation.
Emerging Clinical Applications of Muse Cells
Muse cells’ clinical trials are still in the early stages, but the results so far are promising.
Muse cells show potential to support tissue regeneration at the cellular level. When combined with standard therapies, using MSCs and Muse stem cell treatments may provide a greater impact on chronic conditions.
Below we highlight areas where Muse cells are being tested, mainly through ongoing clinical trials and advanced preclinical studies.
Neurological Repair: Stroke Recovery and Brain Injury Repair
One of the first targets for Muse cell therapy has been stroke, which often leaves patients with permanent brain damage and disability. In 2023, a randomized trial in Japan tested an allogeneic Muse cell product in patients who had suffered a stroke (subacute phase, 2–4 weeks post-stroke).
The results: patients who received Muse cells showed significantly greater motor recovery than those given a placebo. By 4 weeks after infusion, improvements in arm and overall motor function were evident, and these gains persisted through 12 months.
While these efforts are still in their early stages, they indicate that Muse cells as part of mesenchymal stem cell therapies could have a wide range of applications in neurology, from stroke and neonatal brain injury to potentially traumatic brain injury and neurodegenerative conditions.
While Muse cells are still in their early stages, you can learn more about general MSC therapies for post-stroke recovery in our dedicated article.
Read moreHeart Healing
Repairing heart tissue after a myocardial infarction has long been a goal of regenerative medicine. In the first human Muse cell clinical trial (a 2020 Japanese study), heart attack patients with poor ejection fraction (weak heart pump function) received donor Muse cells intravenously within a week after the attack.
The result: After three months of heart disease treatment, the heart’s ejection fraction increased by more than 10% (from ~41% to ~52%), indicating significant functional recovery. Even a 5% improvement is significant; a double-digit increase indicates true cardiac muscle regeneration. There were no arrhythmias or adverse reactions, and follow-ups showed that treated patients had improved heart wall motion and scar size.
MSCs have already shown encouraging results for heart attack recovery. Learn more about how they work in our dedicated article.
Read moreLung Healing
For lung diseases, the damage caused by inflammation and fibrosis in conditions like ARDS (Acute Respiratory Distress Syndrome) has been a focus. During the COVID-19 pandemic, a small clinical experiment in Japan administered Muse cells to patients with severe ARDS. Although detailed results are pending publication, early indications suggested potential benefits in reducing lung inflammation.
Muse cells’ ability—shared with regular MSCs—to modulate immune responses (e.g., calm cytokine storms) and differentiate into airway-lining or blood vessel cells may open up new treatment options for conditions such as ARDS, pulmonary fibrosis, and even severe asthma in the future.
Muscle and Skin Recovery
Muse cells can help in challenging musculoskeletal and skin tissues. A clinical trial in Japan investigated these cells for epidermolysis bullosa (EB), a genetic condition that causes chronic skin ulcerations. Five young adults with EB received Muse cell infusions.
The result: Over the next few weeks, their open skin ulcers shrank significantly (two patients had >50% wound reduction by one month), and by one year the overall wound area was markedly reduced. Patients also reported less pain.
Kim Kardashian, a well-known actress and media person, shared her personal experience with Muse cell therapy. On her Instagram, Kim described the results:
“For me, Muse cells are an innovation that truly changed everything. I regained movement in my shoulder, found relief from chronic back pain, and finally feel like my body is healing again.”
Spinal Cord Recovery
In spinal cord injury (SCI), where lost neurons and connections result in paralysis, Muse cell therapy showed promising results. A 2024 multicenter trial in Japan treated 10 patients with recent cervical spinal cord injuries using donor Muse cells. The primary goal was safety.
The result: no serious treatment-related side effects occurred. But beyond safety, doctors observed meaningful improvements: patients’ motor function scores, as well as daily activity and quality-of-life measures, significantly improved compared to their status before treatment.
At Swiss Medica, we have been helping patients with SCI for over a decade. Learn more about the possible benefits of MSC therapy in our dedicated article.
Read moreChronic Conditions: Diabetes, ALS, Parkinson’s, and More
For chronic disease management, one treatment may not be enough. Ongoing processes like inflammation and tissue damage mean multiple doses could be required. Muse cells show early promise in several chronic diseases, but research is still ongoing and more evidence is needed.
| Condition | What Studies Show | Key Takeaway |
| Diabetes | Early studies suggest Muse cells may help improve organ function, reduce inflammation, and support blood flow. Detailed results are not yet available. | Potential to aid inner organ repair and diabetic complications, but evidence remains limited. |
| ALS & Parkinson’s | In ALS, a 2023 Phase 2 trial tested repeated doses over six months. Safety was confirmed, and results hinted at slower disease progression in treated patients. | Muse cells may protect or replace motor neurons, extending functional ability. Parkinson’s research is still at an earlier stage. |
| Chronic Conditions in General | Ongoing disease processes mean that a single dose may not be enough. In some skin disease cases, one treatment only gave temporary benefit, while repeated dosing showed stronger effects. | Multiple infusions may be required for lasting results. Optimizing treatment schedules is an active area of study. |
Get a free online consultation
Every condition is unique, and treatment possibilities depend on many factors. At Swiss Medica, our medical advisors can help you understand whether stem cell therapy may be suitable for your case. Book your free online consultation by filling out the form.

Medical Advisor, Swiss Medica doctor
Muse Cells and Longevity Therapy
Aging, at its core, involves the accumulation of cellular damage, loss of regenerative capacity, and chronic inflammation. Muse cells can aid in the fight against some signs of aging due to their ability to produce youth-promoting factors.
Researchers are considering enhancing a patient’s own stem cell pool with Muse cells to help with organ maintenance and repair. A lower dose of targeted Muse cells, when combined with regular MSCs, may improve tissue repair and regeneration.
Learn more about anti-aging therapies with MSCs, which naturally include Muse cells, in our article on wrinkle treatments.
Read moreClinical Trials and Evidence: Proof from the Front Lines
This table summarizes the majority of Muse cell clinical trials, which are primarily from Japan, as well as their key findings, safety concerns, and future outlook.
| Condition / Trial | Study Details | Key Results | Takeaway |
| Stroke (2023) | Placebo-controlled trial, 25 patients received Muse cells, 10 placebo, 2–4 weeks after stroke. | Faster and greater motor recovery; by 1 year, better daily function and limb movement vs. placebo. No serious side effects. | Strongest evidence so far: Muse cells improve stroke recovery safely. |
| Heart Attack (2020) | 3 patients, Muse cells IV within 5 days of large MI. | +11% improvement in heart function (EF), reduced scar size, no adverse events, stable at 1 year. | First proof Muse cells can regenerate human heart muscle. Phase 2 is ongoing. |
| Spinal Cord Injury (2024) | 10 patients with cervical SCI, open-label. | Safe; improved motor scores and independence. Some regained partial self-care. | Feasibility shown, recovery beyond rehab expected; larger controlled trials needed. |
| Epidermolysis Bullosa (2021) | 5 patients with severe EB (chronic skin ulcers). | Wounds shrank ~47% on average, less pain, effects lasted 1 year. Donor collagen integrated into skin. | First evidence Muse cells directly regenerate tissue in a genetic skin disease. |
| ALS (2023) | Phase 2, repeated Muse doses monthly for 6 months. | Safe; progression slowed vs historical controls (muscle and breathing decline less steep). | Hints that Muse cells may protect or replace motor neurons; Phase 3 planned. |
The Treatment Process: What It Looks Like in Clinical Trials
Muse cell therapy is still in the testing phase and is being administered in clinical trials with a defined protocol. Here’s what a patient journey in a Muse cell trial generally looks like:
Initial Consultation: Who Qualifies Today
Currently, Muse cell clinical trials recruit patients with specific conditions and within certain time frames or severity grades. The primary focus is on acute or subacute cases (a few days to weeks after injury), with the goal of intervening during a critical period to maintain or restore function. Patients are thoroughly screened before the procedure, including a medical history and baseline imaging/lab tests, to ensure their eligibility and safety.
Clinical trials of Muse cell therapy are evolving and showing encouraging results, and we believe it may become available in our clinic in the near future.
Cell Preparation and Delivery: Current Research Standards
Muse cells are produced in specialized cell processing facilities. Typically, they are isolated from donor bone marrow or other tissue and grown in a controlled environment to ensure that they are the correct types of cells and are not contaminated. The final product used in trials is a sterile suspension of live Muse cells in a predetermined amount.
Muse cells are administered intravenously, just like a standard IV drip. No surgery is needed. Patients sit or lie comfortably as the cell suspension is administered through a vein, typically in the arm. The procedure is generally painless.
Some protocols administer Muse cell therapy as a single infusion, while others deliver a series of infusions over multiple days.
Aftercare: How Patients Are Monitored in Trials
After receiving Muse cells, patients are monitored for months to a year or more. This includes regular visits for physical exams, blood tests, and imaging to check both safety and effectiveness. So far, no tumors, immune rejection, or late complications have been reported.
On the recovery side, patients undergo functional assessments (e.g., motor tests after stroke, neurological exams after spinal injury). Improvements often appear within a few months and have been sustained for at least a year in trials.
At Swiss Medica, we follow strict protocols tailored to each condition to ensure MSC therapy is both safe and effective. Learn more about the steps patients go through before, during, and after treatment.
Read morePotential Risks
Muse cell regenerative therapy, an innovative treatment, is still undergoing safety testing. The majority of them are showing positive and consistent results. However, there are a few things that patients should consider:
- Rare cell population: Muse cells make up about 0,5–1% (sometimes up to 3%) of MSCs, so producing enough for therapy is still challenging and may affect Muse cell therapy cost and availability.
- Experimental status: Outside Japan, Muse therapy is not yet approved and remains limited to clinical trials. Long-term safety (5–10 years) is still being studied.
- General side effects: Like with any stem cell therapy, there may be mild side effects like headaches, redness at the site of the injury, or tiredness. Muse cells are currently considered a safe cell therapy, with consistent results and few side effects.
Muse cells are still in the active research phase but have the potential to move from experimental use to an established therapy. In Japan, they are advancing under the Sakigake fast-track system—similar to the FDA’s Breakthrough designation—with clinical-grade products like CL2020 already in late-stage development.
Outside Japan, most work in Europe and the U.S. remains preclinical, and although some clinics mention Muse cells, their use is still experimental rather than mainstream.
Comparison: When to Use Muse Cells vs. Stem Cells (MSCs)
Muse cells can be thought of as a more specialized and powerful subset of MSCs. Since MSCs are already widely used in clinical practice—and Muse cells are part of this broader stem cell family—it is not accurate to compare Muse cells vs. stem cells like MSCs as if they were separate. As a subpopulation of MSCs, Muse cells also contribute to tissue repair and regeneration.
Future Outlook: What’s Next for Muse Cell Therapy
As research progresses, here are the key areas where patients can expect to see Muse therapy develop in the coming years:
- Scaling up production—developing reliable methods to grow large batches of Muse cells.
- Targeted delivery & combined therapies—combining Muse cells with other advanced therapies, including MSCs, regulatory T cells (Tregs), macrophage-based interventions, or CAR-T therapies.
- Expanding diseases treated—moving beyond stroke and heart attack into chronic heart failure, chronic kidney disease, Alzheimer’s, and Parkinson’s.
- Public awareness & access are expected to grow, with more stem cell clinics beginning to use Muse cells. The Muse cell therapy costs will vary depending on local taxes and operational expenses in each country.
Global Availability: Where Muse Therapy Is Taking Off
As of now, access to Muse cell therapy is largely confined to clinical trials, with Japan being the clear pioneer. Here’s the global landscape:
| Region / Setting | Current Status | Key Notes |
| Japan | Leading in research and clinical trials (stroke, heart attack, spinal cord injury, skin disease). | Supportive regulations (Sakigake fast-track). The Muse product CL2020 in advanced development. Approval may come soon if Phase 3 trials succeed. |
| Other Countries (Europe, North America, etc.) | Mostly preclinical research, some animal studies. | Few labs (e.g., in the US and Italy) are studying Muse cells. Large-scale patient trials not yet launched. International trials likely after Japan’s Phase 2/3 results. |
| Private Clinics | Some advertise “Muse therapy,” but usually not genuine. | Isolating Muse cells is complex; most such clinics use regular MSCs. |
Today’s availability is limited: mostly in Japan, currently in trials, and not yet available to the general public. Tomorrow’s availability is expected to increase significantly, though regulatory timelines may be slow. Over the next 5–10 years, we predict a proliferation of trials on multiple continents as the concept proves its worth.
How to Get Started: Your First Steps Toward Muse Therapy
If you’re thinking about Muse cell therapy, you’re probably wondering what you can do right now to explore this option safely. Here are some steps:
- Stay informed: Check trusted trial registries (like clinicaltrials.gov) for studies using Muse cells. Remember, Muse cells are a natural part of MSC treatments, so before seeking specific Muse-based therapies, it may be worth considering MSC therapy in general.
- Talk to your doctor and consider a second opinion: Ask a specialist familiar with your condition whether any Muse cell studies may be suitable for you. Since not all doctors are aware of stem cell therapies, it’s best to also consult specialists in regenerative medicine.
- Stay connected: Patient groups and communities often share news about new trials and may help you find chances.
- Think about the timing: For short-term conditions, trials may only be open for a short time; for long-term conditions, it might be better to wait for new studies.
- Plan ahead: Trials usually cover treatment Muse cell therapy costs, but travel and follow-up visits may require support and commitment.
Above all, keep an open dialogue with your healthcare team. Muse cells are an exciting new option, but they should complement—not replace—standard treatments. By staying informed and supported, you’ll be well prepared when new opportunities emerge and therapies combining Muse cells with MSCs move closer to becoming a standard option.
Contact us
At Swiss Medica, our regenerative medicine specialists take the time to evaluate your unique case and provide clear, honest guidance—without pressure. During a free online consultation, you can explore whether stem cell therapy may be right for you. Book a free call now.

Medical Advisor, Swiss Medica doctor
Frequently Asked Questions
1. What is Muse cell therapy?
It’s a regenerative treatment that uses Muse cells—a subpopulation of MSCs found in bone marrow and fat—to repair damaged tissues. They can stimulate cell growth and tissue repair.
2. Is Muse therapy available now?
Muse cell therapy is not yet a standard treatment and is currently available only through clinical trials, mostly in Japan. In other countries, research is still at earlier stages. However, MSC therapies—which naturally include Muse cells—are already widely offered.
3. How much does it cost?
In clinical trials, treatment is usually free for participants. Since Muse therapy isn’t on the market yet, there’s no set price.
4. What does the treatment process look like?
Patients receive Muse cells through a simple IV infusion, much like a drip or blood transfusion. No surgery, no anesthesia, and no immunosuppressant drugs are needed.
5. Is it safe?
So far, clinical trials show Muse therapy is safe. No tumors, no immune rejection, and only rare mild side effects have been reported.
6. Who is a candidate?
At present, only people who qualify for clinical trials—such as patients with stroke, heart attack, spinal cord injury, ALS, or rare skin diseases—can access Muse cell therapy. In the future, as more studies are completed, the eligibility criteria are expected to expand.
List of References:
Alanazi RF, Alhwity BS, Almahlawi RM, Alatawi BD, Albalawi SA, Albalawi RA, Albalawi AA, Abdel-Maksoud MS, Elsherbiny N. Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy. Cells. 2023 Jun 21;12(13):1676. doi.org/10.3390/cells12131676
Dezawa M. Comparison of MSCs and Muse cells: the possible use for healthspan optimization. Biogerontology. 2025 Jul 2;26(4):139. doi.org/10.1007/s10522-025-10275-2
Niizuma K, Osawa SI, Endo H, Izumi SI, Ataka K, Hirakawa A, Iwano M, Tominaga T. Randomized placebo-controlled trial of CL2020, an allogenic muse cell-based product, in subacute ischemic stroke. J Cereb Blood Flow Metab. 2023 Dec;43(12):2029-2039. doi.org/10.1177/0271678X231202594
Minatoguchi S, Fujita Y, Niizuma K, Tominaga T, Yamashita T, Abe K, Dezawa M. Donor Muse Cell Treatment Without HLA-Matching Tests and Immunosuppressant Treatment. Stem Cells Transl Med. 2024 Jun 14;13(6):532-545. doi.org/10.1093/stcltm/szae018
Fujita Y, Nohara T, Takashima S, Natsuga K, Adachi M, Yoshida K, Shinkuma S, Takeichi T, Nakamura H, Wada O, Akiyama M, Ishiko A, Shimizu H. Intravenous allogeneic multilineage-differentiating stress-enduring cells in adults with dystrophic epidermolysis bullosa: a phase 1/2 open-label study. J Eur Acad Dermatol Venereol. 2021 Aug;35(8):e528-e531. doi.org/10.1111/jdv.17201
Yamashita T, Nakano Y, Sasaki R, Tadokoro K, Omote Y, Yunoki T, Kawahara Y, Matsumoto N, Taira Y, Matsuoka C, Morihara R, Abe K. Safety and Clinical Effects of a Muse Cell-Based Product in Patients With Amyotrophic Lateral Sclerosis: Results of a Phase 2 Clinical Trial. Cell Transplant. 2023 Jan-Dec;32:9636897231214370. doi.org/10.1177/09636897231214370
Koda M, Imagama S, Nakashima H, Ito S, Segi N, Ouchida J, Suda K, Harmon Matsumoto S, Komatsu M, Endo T, Suzuki S, Inami S, Ueda H, Miyagi M, Inoue G, Takaso M, Nagata K, Yamada H, Kamei N, Nakamae T, Suzuki H, Nishida N, Funaba M, Kumagai G, Furuya T, Yamato Y, Funayama T, Takahashi H, Yamazaki M. Safety and feasibility of intravenous administration of a single dose of allogenic-Muse cells to treat human cervical traumatic spinal cord injury: a clinical trial. Stem Cell Res Ther. 2024 Aug 13;15(1):259. doi: 10.1186/s13287-024-03842-w. Erratum in: Stem Cell Res Ther. 2024 Nov 6;15(1):402. doi.org/10.1186/s13287-024-04044-0
Sato Y, Shimizu S, Ueda K, Suzuki T, Suzuki S, Miura R, Ando M, Tsuda K, Iwata O, Muramatsu Y, Kidokoro H, Hirakawa A, Hayakawa M; SHEILD team. Safety and tolerability of a Muse cell-based product in neonatal hypoxic-ischemic encephalopathy with therapeutic hypothermia (SHIELD trial). Stem Cells Transl Med. 2024 Nov 12;13(11):1053-1066. doi.org/10.1093/stcltm/szae071
Dezawa M. Macrophage- and pluripotent-like reparative Muse cells are unique endogenous stem cells distinct from other somatic stem cells. Front Bioeng Biotechnol. 2025 Mar 27;13:1553382. doi.org/10.3389/fbioe.2025.1553382