Living with multiple sclerosis (MS) often means dealing with changes in mobility, maintaining independence, and managing symptoms that can vary day to day. While standard treatments remain the foundation of care, some patients look for supportive options that might enhance their overall progress.
Stem cell therapy for MS patients explores this possibility by supporting the body’s own capacity for neurological repair while acknowledging its limits: it can enhance recovery—but it is not a cure.
Understanding Multiple Sclerosis
MS is a complex condition, and to understand how it affects the body—and where multiple sclerosis stem cell treatment may offer support—we’ll first look at the fundamentals of how the disease develops.
How MS Affects the Nervous System
MS is an autoimmune disease in which the body’s immune system mistakenly attacks the central nervous system (CNS). This involves two main processes:
- Demyelination occurs when immune cells attack the protective myelin sheath, a protective layer that surrounds nerve fibers, resulting in inflammation and nerve damage.
- Over time, this causes neurodegeneration, with loss of myelin and injury to the nerve axons themselves.
As a result, MS produces a wide range of neurological symptoms—from fatigue and weakness to sensory problems, coordination difficulties, and cognitive impairment. The damage accumulates in the brain and spinal cord, forming lesions or “plaques” that disrupt nerve signaling.
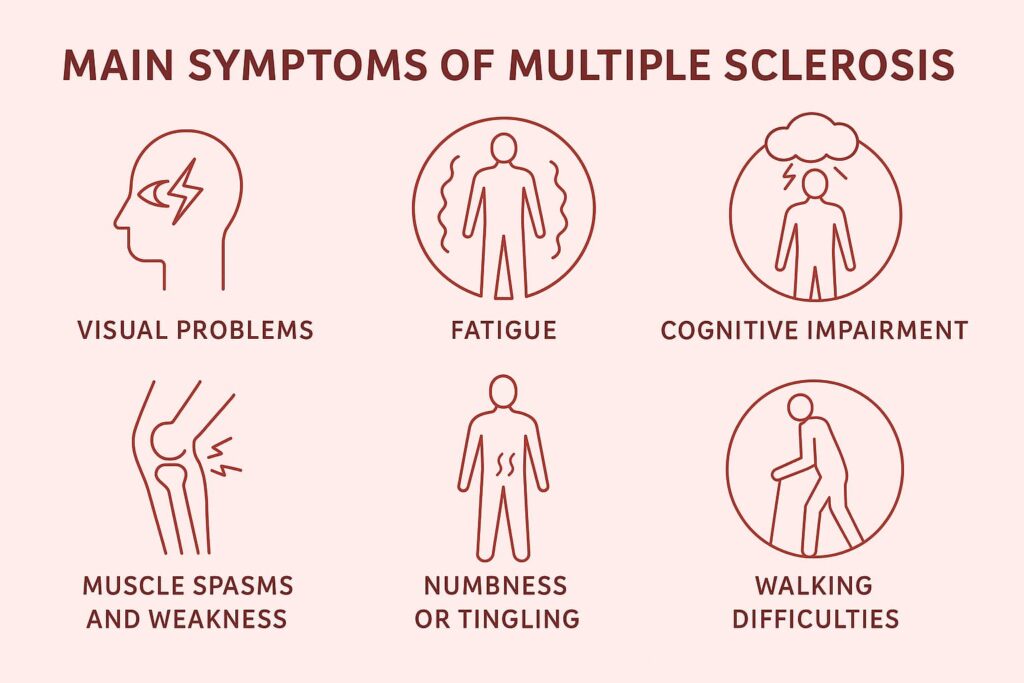
Why Traditional Treatments Have Limitations
Standard disease-modifying therapies (DMTs) for MS primarily work to reduce the frequency of MS relapses and new lesions by suppressing the autoimmune attack. However, medications have important limitations:
- They do not restore lost nerve function and primarily reduce new inflammation.
- Responses differ between individuals, and some forms of MS remain insufficiently controlled.
- They can cause significant side effects, including increased risk of respiratory and urinary infections and gradual decreases in immunoglobulin levels.
Conventional therapies may not be enough to protect the nervous system from further damage. This is why, in addition to standard treatment, some MS patients seek regenerative strategies like stem cell therapy for MS.
What Stem Cell Therapy for MS Is
Patients often first want to know the effectiveness of stem cell treatment for MS and how it works. Here’s a brief overview of MS regenerative therapy.
How Does Stem Cell Therapy Work for MS: Regenerative Principles Behind Multiple Sclerosis Stem Cell Treatment
Stem cell therapy for MS focuses on supporting the body’s repair mechanisms. The most studied method uses mesenchymal stem cells (MSCs), obtained from bone marrow, placenta, or umbilical cord tissue. After administration, MSCs move toward inflamed or injured areas and contribute to recovery by several key actions.
Principle 1. Supporting Natural Repair Mechanisms
Mesenchymal stem cells for MS may help protect and repair myelin by altering the environment in which it is repaired, rather than replacing damaged myelin. They release growth factors that may encourage the brain to produce new myelin while also clearing inhibitory debris and creating a more supportive setting for remyelination.
Principle 2. Reducing Inflammation and Abnormal Immune Activity
MSCs may help reduce inflammation in injured tissue by inhibiting the production of pro-inflammatory compounds, which could lead to fewer relapses and fewer new MRI lesions.
Principle 3. Modulating the Immune System
MSCs can help reduce the autoimmune response that attacks myelin sheaths. Unlike broad immunosuppressants, they appear to adjust immune activity rather than suppress it completely.
The Result: Potential Influence on Symptoms and Disease Progression
By helping with repair and lowering inflammation, MSC therapy may lead to small improvements in function and greater stability, like better mobility, less fatigue, better bladder control, or small gains in cognitive processing. While benefits are variable, the therapy may help stabilize symptoms and reduce inflammatory activity, even though reversing MS with stem cells is not currently possible.
If you’d like a deeper look at how MSCs work in the body, our detailed article explains the mechanisms supported by scientific studies and observations in MS and other chronic conditions.
Read moreSecure vs. Unregulated Approaches in Stem Cell Therapy for Multiple Sclerosis
Patients considering therapy with stem cells for neurological disorders should understand the difference between medically regulated treatments and unregulated interventions. Regulated programs follow established protocols, use clinically tested cell types, and maintain strict safety and oversight. By contrast, some clinics worldwide may advertise their products as “new stem cell treatments for MS”, but often:
- Do not follow established medical protocols. They may skip proper eligibility evaluations or use incorrect cell dosages.
- Use untested cell types. For example, use embryonic stem cells or administer cells of questionable quality.
- Use aggressive advertising. Some providers may promise unrealistic cures.
- Operate with minimal oversight. Some clinics might not screen cells for contamination.
The Effectiveness of Stem Cell Therapy for Multiple Sclerosis
Patients must distinguish hematopoietic stem cell transplantation (HSCT) from MSC therapy, as these products have been studied separately.
Hematopoietic Stem Cell Transplant (HSCT)
Uses high-dose chemotherapy to eliminate the existing immune system and then rebuild it with the patient’s own blood-forming stem cells. Although HSCT can be effective for aggressive relapsing MS, with many patients achieving long-term remission, it is an intensive procedure with significant risks. For this reason, it is generally reserved for younger patients with severe, treatment-resistant MS and is offered mostly in specialized centers.
MSC Therapy
Uses mesenchymal stem cells for multiple sclerosis to help modulate immune activity and support tissue repair.
Key findings from clinical research:
- In a phase II trial, most patients were more likely to have a stable condition than the placebo group. At one year, 58.6% of patients who received MSC treatment showed no signs of disease activity, while only 9.7% of patients who received a placebo did. Furthermore, MSC-treated patients developed fewer new MRI lesions and showed modest gains in walking speed and arm function.
- Studies with repeated mesenchymal stem cell injections for MS reported safety and signs of clinical stabilization or improved muscle strength.
- A 2023 meta-analysis found reduced inflammatory activity and modest functional improvements.
What realistic improvements may patients expect from mesenchymal stem cells for multiple sclerosis? Based on published research, it is:
- Potential stabilization of the disease (fewer relapses or new lesions),
- Reduced disability progression,
- MS symptom improvement—decreased fatigue, increased mobility, and improved bladder function, among others.
The results can vary—some patients may see significant gains, while others may experience mild to moderate improvements.
Who May Be a Candidate for Stem Cell Therapy for MS
| Who May Qualify for Multiple Sclerosis Stem Cell Treatment | Who May Not Qualify for MS alternative treatment options |
| Patients with very mild, well-controlled MS where added risk may not be justified; adults with relapsing-remitting or active secondary progressive MS that continues to show inflammation despite standard treatments | Individuals with very advanced disability with low potential for recovery |
| Typically ages 18–65, with broader eligibility possible after evaluation | Patients with severe organ dysfunction or active infections |
| Adequate general health aside from MS, with no uncontrolled medical issues | Those with active cancer, severe immunosuppression, certain blood disorders, or pregnancy |
Before being approved for neuroregenerative medicine, patients typically undergo a thorough medical evaluation. This might include review of medical records (MRI scans, MS history, previous treatments), lab tests to ensure no hidden infections or blood issues, and imaging like a chest X-ray or EKG to check general health.
Get a free online consultation
If you want to know if stem cell therapy is right for you, schedule a free online consultation with a regenerative medicine specialist at Swiss Medica. Our team will provide you with a clear picture based on your specific case, including realistic expectations and outcomes.
What Stem Cell Therapy for MS Looks Like
Swiss Medica is one of the world’s largest clinics offering stem cell therapy in Serbia. We have over 14 years of experience treating MS and other neurological conditions. Treatment is typically delivered through a multi-step program:
- Diagnostics: Before treatment, our doctors review MRI scans, test results, and medical history through online consultations. It helps understand the patient’s current condition and choose the most suitable cell source—most often donor MSCs from umbilical cord or placenta. Autologous bone marrow–derived MSCs may be considered, but in some cases they cannot be used due to contraindications that can be identified during the consultation. All cell products are tested for sterility and quality in our in-house laboratory.
- MSC infusion: MSCs are given intravenously, intrathecally, or sometimes through both routes. During each procedure, the patients are watched closely.
- Supportive regenerative procedures: As part of our comprehensive protocol, we may employ additional cell-based products:
- Neural stem cells (NSCs) to support myelin repair,
- Exosome or secretome therapy to deliver signaling molecules that aid recovery,
- Macrophages to help clear debris and reduce inflammation,
- Intracellular Metabolism Recovery (IMR)—a personalized mix of vitamins, minerals, amino acids, antioxidants, and trace elements to support overall effect.
- Physiotherapy integration: While the stem cells do their work internally, physiotherapy helps the body re-learn functions and strengthens neural pathways. During the stay, our patients may receive physiotherapy, kinesiotherapy, massage, and other rehabilitation methods.
- Follow-up: After discharge, our team provides structured follow-up, including check-ins at 3–6 months and guidance on maintaining progress. Additional treatments may be recommended based on individual outcomes.
Potential Benefits of Stem Cell Therapy for MS Patients Often Seek
For MS patients, outcomes of stem cell therapy can vary from mild symptom relief to noticeable improvements in mobility and quality of life.
Fatigue and Mobility Support
Patients frequently report decreased fatigue and increased mobility as a result of reduced inflammation and improved nerve function.
Quality-of-Life Improvements
Patients mention broader quality-of-life benefits such as improved daily functioning, stable mood, better sleep, and increased independence in routine activities.
Neurological Function, Coordination, and Comfort
Some patients report better coordination, sensory symptoms, or overall comfort in feeling and controlling their bodies.
— A patient from Germany
“After more than 20 years with MS, I didn’t expect much—but three days after starting stem cell therapy, my pain disappeared. It stayed gone for the entire year. I regained strength in both sides of my body, my fatigue eased, and I could go out after work again, enjoy parties, and tolerate noise for the first time in years. Coming back for the second program, with neural stem cells and new procedures, gave me even more stability. For me, it has been worth it.”
You can find more testimonials on our YouTube.
Safety Considerations
Most studies indicate that MSCs are generally safe and may offer benefits in MS, particularly in reducing inflammation and supporting functional stability. They are generally well tolerated without the need for immunosuppressive drugs. Most side effects are mild and temporary—like headache, low-grade fever, or brief fatigue.
Safe MSC therapy means the cells were processed in a Good Manufacturing Practice (GMP) facility, tested for bacteria, viruses, and endotoxins, and checked for viability and identity. Swiss Medica’s GMP-certified laboratory ensures that all cell products pass rigorous quality controls.
Look inside our lab and see how we carefully prepare and test every stem cell product at Swiss Medica.
Read moreSuccess Rates and Long-Term Outcomes
The most important outcomes can be stability, fewer relapses, and improved daily functioning, but current research shows that looking at stem cells for MS as a cure is unrealistic. MSC therapy aims to slow the disease and ease symptoms rather than reverse MS entirely.
Long-term results vary by the type and stage of MS, the amount of existing damage, and the treatment protocol used. Initial effects typically appear within the first three months. The cells themselves remain active for about 6 months, and after they naturally complete their lifespan, some of the therapeutic benefits may persist for an additional 6–12 months.
How Stem Cell Therapy Compares With Other MS Treatments
In this table, we compare various approaches, specifically HSCT vs. stem cell therapy.
| Aspect | Standard DMTs | HSCT | MSC Therapy | Physio & Supportive Care |
| Goal | Reduce immune attacks and slow new lesion formation | Halt aggressive MS by modulating the immune system via chemo and stem cell reinfusion. | Modulate the immune system and promote repair of tissues. Works better in addition to standard therapies. | Maintain and improve existing function, and manage symptoms. |
| Efficacy | Proven relapse reduction. | Efficacy in aggressive MS. | Potentially reduced disease activity and some functional improvements. | Improves mobility and daily function. |
| Safety | Varies by drug. | Significant chemo-related risks. | Mostly mild, short-term side effects. | Generally safe with guidance. |
| Availability | Widely available. | Limited to specialized centers and requirements. | Accessible via clinical trials or private clinics in some countries. | Standard part of MS care. |
What Is the Cost of Stem Cell Therapy for MS?
The stem cell treatment cost can vary depending on the number of doses, cell source, length of stay and included therapies, regional regulations, and operational costs.
| Where Can I Get Stem Cell Treatment for MS | Typical Cost of Stem Cell Therapy for MS |
| United States | $15,000–$30,000+ |
| Europe | €10,000–€40,000 |
| Serbia | €7,000–€31,000* |
| Latin America & Asia | $10,000–$30,000 |
Stem cell therapy in the USA is offered mostly through clinical trials or private clinics, while stem cell therapy in Europe tends to be expensive due to inflexible regulations and facility costs. That is why some patients also look into the cheapest countries for stem cell treatment in Latin America or Asia, like Mexico or India.
However, lower prices can carry a higher risk of poorly regulated clinics. Serbia, and particularly Swiss Medica, offers a safer alternative: proven, medically supervised stem cell programs at more accessible prices.
*Prices are indicative and based on 2026 estimates; they may vary depending on condition severity and required cell quantity.
How to Choose the Best Clinic for Stem Cell Treatment for MS
With numerous clinics worldwide offering stem cell treatments, selecting the right clinic is crucial. Clinics differ in expertise, protocols, safety measures, and cost of stem cell therapy for MS. Here are important factors to consider:
- Medical expertise: Look for a clinic that focuses on neurology. MS is a complex disease, so you want doctors who understand MS pathology and have treated neurological patients. Furthermore, MS treatment abroad should follow a personalized protocol rather than a one-size-fits-all approach.
- Safety: Look for GMP-certified labs and clear risk explanations from doctors.
- Track record: Check consistent patient feedback and published clinical practices.
- Cost: Price matters, but the lowest or highest cost doesn’t guarantee quality. What you need is good value—a fair price for safe, well-run care. Always request a detailed quote and check exactly what is included.
- Patient support: Pay attention to how the clinic communicates with you from the start. If you feel pressured or the communication appears evasive, it’s a red flag.
Why Patients Choose Swiss Medica
Swiss Medica has been treating patients with MS and other neurological disorders (like Parkinson’s, ALS, stroke) for more than 14 years. Patients from all over the world prefer it for its specific benefits:
- Honest communication: From the first contact, our specialists explain what results are realistic for your specific case, rather than giving general promises.
- Personalized protocols: Treatment plans are adapted to each MS type and adjusted for factors like disease duration and symptom profile.
- Comprehensive regenerative approach: MSC therapy is used together with other treatments like exosomes/secretome, macrophages, and supportive therapies.
What to Expect During Your Stay
Beyond medical expertise, our modern hospital offers an all-inclusive setup that minimizes stress and supports recovery:
- Accessibility: Wheelchair-friendly facilities, ramps, and mobility support.
- Accommodation: Private, accessible rooms with space for a caregiver.
- Meals: Nutritious meals with dietary needs accommodated.
- Daily schedule: Structured days with medical checks, infusions, and planned therapies, plus time to rest.
- Airport pickup: A driver meets patients on arrival and brings them directly to the hospital.
- Language support: Staff speak English; translators can be provided when needed.
- Aftercare: Clear discharge instructions and ongoing contact with a doctor.
- No hidden costs: Packages include treatments, accommodation, meals, and transfers.
At Swiss Medica, multiple sclerosis stem cell treatment is supported by skilled doctors, comfortable spaces with wheelchair accessibility, and an in-house lab that thoroughly prepares each cell product.
How to Start Your Treatment Journey
You start by sending your medical records and having a free online consultation. If you decide to move forward, our team reviews your case, creates a personalized plan, and confirms the details.
Contact us
To learn about your specific case, book a free online consultation with our medical advisor. We will review your history, check your suitability, and explain what results you may reasonably expect.
Frequently Asked Questions
1. Can stem cell therapy cure MS?
No—the use of stem cells for MS as a cure is not supported by current evidence, but treatment may help reduce inflammation, stabilize the condition, or ease certain symptoms.
2. How long do the results last?
Improvements can last from several months to a year or longer, though effects vary, and some patients choose repeat treatments when benefits begin to fade.
3. Where can I get stem cell treatment for MS?
Stem cell therapy for multiple sclerosis is available in several settings around the world.
Patients can access stem cell therapy for multiple sclerosis in the US mainly through clinical trials or specialized hospital programs, usually with strict eligibility criteria.
Stem cell treatments for multiple sclerosis in Europe are offered both in university hospitals (mainly HSCT) and in specialized regenerative medicine clinics (mainly MSC-based therapies). Costs can vary widely between countries, with Western Europe typically more expensive than Eastern and Central Europe.
Serbia offers more accessible options, providing European-level treatments at a lower price point, while still benefiting from experienced medical teams and modern clinical standards.
4. Is it safe for elderly patients?
MSC therapy can be suitable for older adults if their overall health is stable, with eligibility decided case by case.
5. How many stem cell sessions do MS patients need?
Most start with one treatment cycle and consider additional sessions only if symptoms return or improvements decline over time.
List of References:
Petrou P, Kassis I, Levin N, Paul F, Backner Y, Benoliel T, Oertel FC, Scheel M, Hallimi M, Yaghmour N, Hur TB, Ginzberg A, Levy Y, Abramsky O, Karussis D. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020 Dec 1;143(12):3574-3588. doi.org/10.1093/brain/awaa333
Jason A. Berard, Mark S. Freedman, Ruth Ann Marrie, James J. Marriott, Harold L. Atkins, David Szwajcer, David. W Courtman, Simon Thebault, Lisa A.S. Walker,
Mesenchymal stem cell therapy and cognition in MS: Preliminary findings from a phase II clinical trial, Multiple Sclerosis and Related Disorders, Volume 61, 2022, https://doi.org/10.1016/j.msard.2022.103779
Sheikhi Kamran , Ghaderi Salah , Firouzi Hassan , Rahimibarghani Sarvenaz , Shabani Ehsan , Afkhami Hamed , Yarahmadi Aref. Recent advances in mesenchymal stem cell therapy for multiple sclerosis: clinical applications and challenges. Frontiers in Cell and Developmental Biology, Volume 13 – 2025. https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2025.1517369
Harris VK, Stark J, Williams A, Roche M, Malin M, Kumar A, Carlson AL, Kizilbash C, Wollowitz J, Andy C, Gerber LM, Sadiq SA. Efficacy of intrathecal mesenchymal stem cell-neural progenitor therapy in progressive MS: results from a phase II, randomized, placebo-controlled clinical trial. Stem Cell Res Ther. 2024 May 23;15(1):151. doi.org/10.1186/s13287-024-03765-6
Wang, Y., Yi, H. & Song, Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther 12, 545 (2021). https://doi.org/10.1186/s13287-021-02609-x
MD, Pediatrician, Regenerative Medicine Specialist