Introduction
Vision is perhaps the most crucial tool of comprehension of the world for human beings. This theory was perfectly illustrated by the great English poet, John Milton, who put the following exclamation into the mouth of his hero, the biblical Samson, blinded by his enemies:
“O loss of sight, of thee I must complain!
Blind among enemies, O, worse than chains,
Dungeon, or beggary, or decrepit age!
Light, the prime work of God, to me is extinct,
And all her various objects of delight
Annulled, which might in part my grief have eased.”
(a fragment of the poem “Samson Agonistes” by John Milton).
Vision disorders are often harder to bear than other impairments, as sight is the dominant sense in humans. The importance of vision is reflected in the complexity of the eye structure. Vision works in the following way, human eyes capture rays of light, transform them into neuronal signals and transfer them to the brain where they are processed into images.
Light is perceived by the retina, which is the light-sensitive layer at the back of the eye. Here is a brief description of the neuronal cells of retina:
Retinal neurons may die due to an injury or pathological process in the eye. As their loss can’t be restored, it may lead to irreversible visual impairment or blindness, which is the primary cause of incurable reduced vision and blindness worldwide. Currently, scientists are focused on attempts to regenerate photoreceptor cells and create a new therapeutic approach to vision impairment.
Mesenchymal Stem Cells and Their Role in Retinal Reparation
Stem cells are the specific type of cells that provide standard renewal of tissues in the human body and activate regeneration processes in response to injury. They divide and differentiate into specialised cells, which are responsible for functional features of each organ and tissue. Typically, they are present in all tissues in our body. Scientists have learned how to extract and cultivate them and then use them for the treatment of various diseases and conditions associated with loss of cells, cell degradation and tissue damage. This approach is called cell-based or regenerative medicine. For practical purposes, mesenchymal stem cells (MSCs) are the most useful, as it is easy to isolate them from various sources (bone marrow, fat tissue, etc.) and cultivate them to the required amount.
MSCs have already shown their therapeutic potential in animal models and clinical studies in humans for the treatment of numerous degenerative disorders, including those of the eye. The ability of MSCs to modulate the immune system and inflammatory response, plus their role in neuroprotection has been proven.
There are several explanations for MSCs’ role in treating ocular disorders. MSCs can directly develop into retinal neuron cells in vitro. When introduced into the body, they stimulate tissue repair by protecting it from programmed cell death and/or modulate inflammation, (inflammation is the underlying pathological process typical of many disorders), and form new blood vessels through secretory molecules. It has been shown to date that MSCs produce and secrete growth factors, cytokines, mRNA and other biologically active molecules which provide its functional benefits.
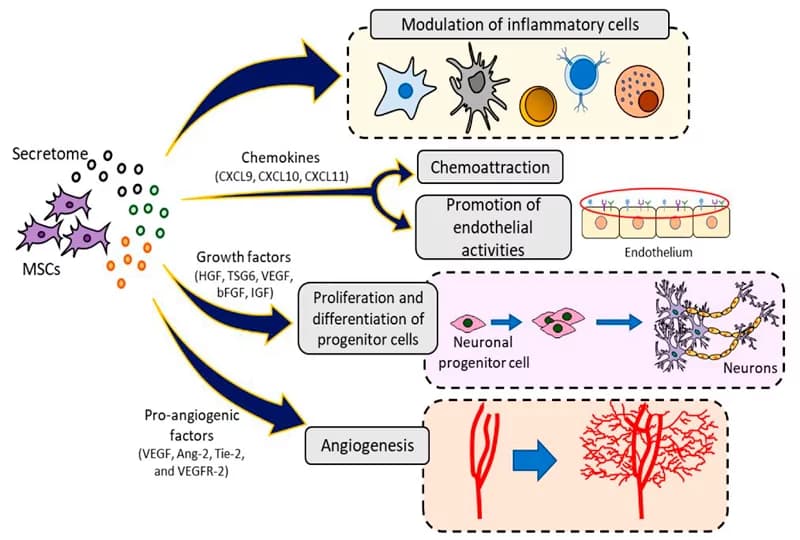
What Eye Diseases Can Stem Cells Benefit?
The above-listed properties of MSCs, including nerve growth factor (NGF) and multiple neurotrophic factors, show that they may be beneficial for the treatment of vision disorders. A list of the registered clinical trials using MSCs for this purpose is presented in Table 1 (source).
| Disease | Cell Type | Source | Route of Administration | Clinical Trial Number |
| Optic nerve inflammation; retinitis pigmentosa; macular degeneration; glaucoma | BM-MSC | Auto | IVIT; iv.; ST; IO | NCT02016508 |
| Macular degeneration | BM-MSC | Auto | IVIT | NCT02016508 |
| Macular degeneration | BM-MSC | Auto | IVIT | NCT01518127 |
| Optic nerve inflammation | NS | Auto | iv. | NCT02249676 |
| Optic nerve inflammation | WJ-MSC | Allo | NS | NCT01364246 |
| Limbal stem cell deficiency | BM-MSC | Allo | NS | NCT01562002 |
| Behcet’s disease | BM-MSC | Auto | IVIT | NCT00550498 |
| Ischaemic retinopathy | BM-MSC | Auto | IVIT | NCT01518842 |
| Retinitis pigmentosa | BM-MSC | Auto | IVIT | NCT01068561 |
| Retinitis pigmentosa | BM-MSC | Auto | IVIT | NCT01560715 |
| Retinitis pigmentosa | BM-MSC | NS | IVIT | NCT01531348 |
| Retinitis pigmentosa | BM-MSC | Auto | NS | NCT01914913 |
Contact us
Get a free online consultation to discover the results you can expect from stem cell treatment.

Medical Advisor, Swiss Medica doctor
Retinitis Pigmentosa and Macular Degeneration
Retinitis Pigmentosa is a genetic disorder; it is manifested by a slow loss of night and side vision. In age-related macular degeneration, a patient loses their central sight. Photoreceptor cell death causes both diseases. In animal model studies, MSCs showed the ability to induce differentiation in photoreceptor cells. They can also protect photoreceptors and other retinal cells from degradation. The completed clinical study in patients with the most common form of retinitis pigmentosa (Usher syndrome) demonstrated improvement in visual acuity in 80% of people’s eyes.
Glaucoma
Glaucoma is an incurable disease which comprises various optic neuropathies. The number of neurons, called retinal ganglion cells (RGCs), continuously decreases, which results in vision impairment. The process may be slowed down by medical treatment and/or surgery; but, the degradation of these cells cannot be stopped entirely. Stem cells, including MSCs, clearly demonstrate their ability to protect RGCs and increase their survival in both model studies and humans.
Diabetic Retinopathy
In diabetic patients, elevated levels of blood sugar often cause damage to the blood vessels, including those located in the retina. Diabetic retinopathy (DR) is associated with the inflammation and death of neuronal cells and damage to the small vessels of the retina. MSCs from different sources have been explored as a possible treatment for DR, with promising results in both preclinical and clinical studies. A study in 2018 demonstrated that patients with non-proliferative DR (without abnormal blood vessel growth) had a significant improvement in vision parameters.
Optic Neuritis
The inflammation of the optic nerve cells is called optic neuritis. It includes the loss of the nerve fibre’s myelin shield and typically manifests as sudden central vision loss in one eye and pain with eye movement. The ability of stem cells to protect and rebuild the myelin layer in the central nervous system has been intensively studied in multiple sclerosis, and a positive effect on patients has been confirmed in clinical trials.
Stargardt’s Macular Dystrophy
Stargardt’s Macular Dystrophy or Stargardt disease is another incurable, inherited retinal disorder, caused by the mutation (change) of the ABCA4 gene. If both a mother and a father have this mutation, their child may inherit this disease. Patients experience vision loss at an early age, light sensitivity and colour blindness. According to the National Eye Institute of the National Institute of Health (NIH, USA), stem cell-based therapy has shown promising results for Stargardt disease in clinical trials.
Inflammation in Retinal Disease
In an experimental dry eye study, MSCs suppressed inflammation and protected the ocular surface. Also, MSCs demonstrated neuroprotective and protective effects on retinal ganglion cells in a glaucoma model.
Chronic and Recurrent Autoimmune Uveitis
In experimental models on animals, later administration of MSCs during recurrent experimental autoimmune uveitis (rEAU) prevented structural and functional damage to the retina. However, treatment during the early stage of rEAU produced more satisfactory clinical efficacy. Additional therapeutic advantage was achieved when therapy was administered twice with longer intervals between.
Neuromyelitis Optica Spectrum Disorder
In 2016, a group of patients was treated with MSC infusions for neuromyelitis optica spectrum disorder (NMOSD). The observed benefits of MSC infusion included a reduction in the frequency of the relapse, mitigation of neurological disability with neural structures in the optic nerve and spinal cord in recovering patients with NMOSD throughout a two‐year observational period. The safety of MSC infusions was also confirmed.
Optic nerve atrophy
In a study published in 2019, MSCs derived from bone marrow were transferred either directly to the optic nerve or in close to the optic nerve and retinal ganglion cell layer. 83.3% of patients with dominant optic atrophy, who underwent the treatment, showed visual improvement that was maintained for up to 24 months. Also, the improvement was observed in both eyes.
How is the Procedure Performed?
Before a patient receives treatment, they are carefully examined and have specific tests carried out. After a comprehensive checkup, our specialistы select the correct therapy, based on the individual needs of the patient and their state of health.
In general, the following steps are performed:
- Stem cell collection (using a biopsy, if the patient’s autologous cells are to be used).
- Cultivation of the collected cells, to increase the cell product to the appropriate quantity.
- The resulting cell product is administered to the patient (via IV drip and/or locally to the eye area).
Get a free online consultation
Contact us to receive detailed information about stem cell treatment for your disease.

Medical Advisor, Swiss Medica doctor
What Are the Results? Patient’s Review
Watch the testimony of Sadhana J., a patient from Singapore who underwent stem cell treatment for uveitis at a Swiss Medica clinic. After several surgeries on each eye, immunosuppressive and steroid therapy, regular eye drops, she was still experiencing vision deterioration. Within one month of treatment using stem cells, improvements in visual acuity were noticed – she could read smaller fonts, for example. The clinical changes that lie behind this result are:
- Suppression of the autoimmune aggression of the disease.
- Reduction of inflammation.
- Improved microcirculation in the affected tissues.
- Blocking of degenerative processes, and stabilisation of her current condition.
- Activation of the body’s stem cell pool and their regenerative potential.
Is MSCs Treatment Safe?
The safety of using MSCs has been demonstrated in animal models. In these studies, no formation of tumours was seen as a result of the cell administrations. Some researchers even demonstrated the anticancer properties of MSCs. A record of the clinical investigations into ‘adult’ MSCs (non-foetal and non-embryonic), for diverse indications, has been maintained for several years. So far, MSC administration has not caused tumourigenesis or any other serious side effects in patients.
Conclusion
Vision disorders are a group of diverse diseases and conditions, both hereditary and acquired, which result in pathological changes of the eye structures, mostly the retina. In many patients, the degradation of the affected cells is irreversible, and vision cannot be recovered. However, the development of cell-based treatment approaches gives hope of an improvement in their vision to these patients.
Contact us
Find out whether you would benefit from stem cell treatment in your case.

Medical Advisor, Swiss Medica doctor
Medical Advisor, Swiss Medica doctor







