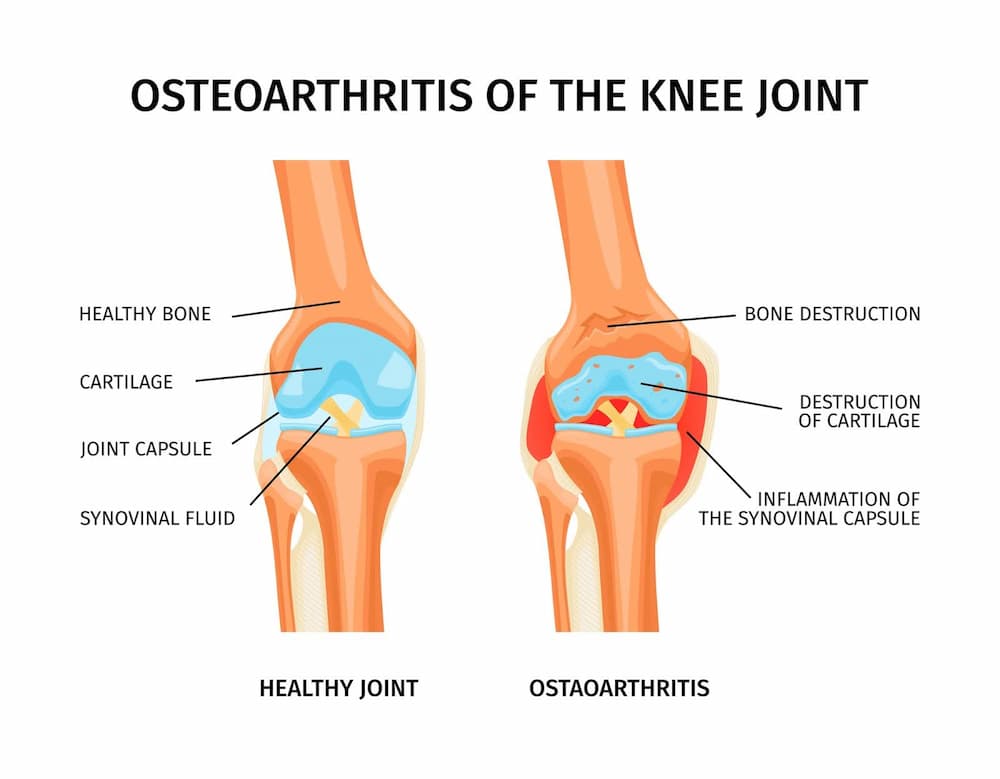
More than 500 million people worldwide suffer from cartilage damage and osteoarthritis, a condition that is generally linked to getting older.
Cartilage is a connective tissue that covers the ends of bones at joints and provides a lubricated surface so we can move without pain. Over time, or because of an injury, the cartilage undergoes changes. This can lead to a condition called osteoarthritis, which causes pain and makes it hard to move.
With stem cell cartilage regeneration, individuals with cartilage damage are given a ray of hope as their pain is relieved and mobility improves following therapy. In this article, we aim to explain how stem cell cartilage repair works.

The Role of Stem Cells in Cartilage Regeneration
Research on stem cells and osteoarthritis is advancing rapidly, promising revolutionary treatment breakthroughs.
Types of Stem Cells Used in Cartilage Regeneration
The use of stem cells to regenerate cartilage has shown proven advantages, particularly in the context of cartilage injuries and osteoarthritis.
Each type of these cells has its own special properties. Understanding the characteristics and sources of them is essential for effective stem cell cartilage treatment.
- Pluripotent stem cells e.g. embryonic stem cells—usually obtained from the embryo and hence have ethical issues associated with them.
- Induced pluripotent stem cells.
- Mesenchymal stem cells from bone marrow, adipose tissue, synovial fluid, placenta, and umbilical cord.
- Cartilage progenitor cells.
Mesenchymal stem cells are most commonly used for cartilage regeneration due to their ability to differentiate into chondrocytes, which are the cells that make up cartilage.
Stem Cell-Based Therapies for Cartilage Repair
Among the different therapies available for stem cell cartilage regeneration, these two are most important:
- In autologous chondrocyte implantation, a person’s chondrocytes from a different site of the joint are taken and expanded in a lab to be later implanted into the damaged area. This technique is used to treat localized cartilage defects and has shown promise.
- During mesenchymal stromal-cell-based therapy, stem cells are obtained from bone marrow and adipose tissue. These cells are then grown in a lab, where they form groups of cells and can change into different types of cells, like cartilage and fat cells.
Mechanisms of Stem Cell-Induced Cartilage Regeneration
Let’s take a closer look at the mechanism of stem cell cartilage repair. It involves the following steps:
- The joint space is infused with stem cells.
- Mesenchymal stem cells migrate from the joint cavity into the damaged cartilage.
- Chondrogenic factors are secreted at the site of damage.
- Mesenchymal stem cells help other stem cells turn into chondrocytes, which play a vital role in creating cartilage.
- As the developing chondrocytes repair the damaged and diseased cartilage, joint discomfort decreases, and mobility gradually improves.
Get a free online consultation
Please, contact our medical advisor to discuss your health condition with a specialist in regenerative medicine. You can also leave your contact details for a callback. It is free and confidential.
Stem Cell Treatments for Cartilage Repair
Stem cell treatment for cartilage repair typically requires some planning and preparation. From selecting the most suitable stem cell type to designing a tailored treatment approach, careful consideration of various factors is necessary.
Preparing for Stem Cell Cartilage Repair Procedures
Once the doctor determines that the stem cell treatment is appropriate for the patient, they give instructions on how to prepare for the procedure.
- Doctors may conduct various tests, such as a full blood count, to rule out any ongoing infections. The procedure may be postponed until these issues have been resolved.
- To get the most out of cell therapy, it’s best to steer clear of NSAIDs before and after the treatment. In a study on animals with joint arthritis, researchers discovered that using NSAIDs after injections made the cell treatment less effective.
Step-by-Step Process of Stem Cell Injection
Stem cell injection procedures involve a systematic approach to ensure optimal results.
- Extraction of Stem Cells: Healthy stem cells are extracted from the patient’s body or a donor’s body. The most common sources are bone marrow, adipose (fat) tissue, or umbilical cord.
- Preparation and Testing: The extracted stem cells undergo processing and rigorous testing to ensure they are healthy and safe for injection. In some cases, they may be cultured in a lab to increase their numbers. This preparation process is crucial to ensuring an adequate number of viable stem cells are available for the treatment.
- Injection Procedure: The prepared stem cells are injected directly into the damaged area of the joint.
- Tissue Regeneration: Once injected, the stem cells work to repair the damaged tissues. They have the potential to differentiate into the required cell types and integrate into the existing tissue. Also, they secrete factors that promote healing and recruit other cells to aid in that process.
Recovery and Rehabilitation After Treatment
After undergoing stem cell therapy for cartilage restoration, patients may be discharged from the hospital on the same day. Usually, they have a swift and rapid recovery and can return to work and daily activities within a couple of days.
Some discomfort at the injection site is common for up to two weeks, but it can usually be managed with over-the-counter pain medication. While the rehabilitation program is tailored to the individual, full recovery usually takes four to six months.
This recovery process can be divided into several stages:
- The initial period (0–3 days): Once treatment has been given, the initial goal is to manage the discomfort and protect the affected area. It is recommended to move the joint minimally.
- Days 4–14 post-treatment: It is advisable to move fully during this era. Exercises are done to improve tissue tolerance and local blood circulation.
- 2-4 weeks post-treatment: More strengthening and endurance workouts are recommended 2-4 weeks after treatment.
- 5-10 weeks post-treatment: During this stage, the patient should aim to engage in full strengthening and endurance activities.
Benefits and Outcomes of Stem Cell Cartilage Repair
Stem cell cartilage repair leverages the body’s natural regenerative capabilities to repair damaged tissues and organs. For many patients, it offers a potential alternative to traditional treatments.
Improved Joint Function and Pain Relief
Stem cell cartilage regeneration leads to improved joint function and pain relief. The latter can begin as early as six weeks after the procedure.
Long-Term Benefits and Sustainability
Research conducted at the 5-year follow-up found a significant improvement in pain and joint function when compared to the baseline. What sets stem cell therapy apart from other forms of treatment is its long-term benefits with almost no acute side effects.
Patient Success Stories and Testimonials
Stem cell regeneration therapy has seen many success stories, including that of the renowned Argentinian footballer Sergio Aguero. He underwent the therapy three years ago and stands as one of its most notable success stories in regenerative medicine.
Swiss Medica’s excellent patient outcomes are attested to by the hundreds of success stories, particularly with stem cell treatment for arthritis.
One of the patients treated at Swiss Medica mentioned, “I’ve regained full mobility since being at the clinic, but initially, there was no movement.”
Ongoing Research and Advancements
Numerous researchers have looked for additional strategies to enhance the success of stem cell cartilage regeneration.
Current Trends in Stem Cell Research for Cartilage Regeneration
The research shows that the strength of the rebuilding muscle significantly increases when animals with muscular atrophy are treated with stem cells carrying methyltransferase inhibitors. This highlights the potential of MSC therapy for cartilage repair within the body.
Furthermore, researchers are continuously studying which types of stem cells work best for regenerating cartilage. This includes finding stem cell-like cells in cartilage and other tissues with high stem cell content, like dental pulp and growth plates in adolescents.
Is Stem Cell Cartilage Repair Right for You?
Candidacy and Eligibility for Stem Cell Treatments
Stem cell cartilage therapy is a viable option for most individuals with cartilage injuries. Some people often seek stem cells to regrow cartilage, but in fact, the use of stem cells for cartilage regeneration is still an area of active research and clinical development.
Additionally, one must consult a doctor who is experienced with the procedure to determine whether the treatment is right in the particular case.
Consultation and Evaluation: Assessing Your Joint Health
A significant amount of morbidity can be avoided with an early evaluation of joint pain. Even in the early stages of pain relief, basic activities can be beneficial—it can stop an illness from getting worse.
Before the procedure, it is crucial to undergo a comprehensive assessment and consultation. At Swiss Medica, we offer these services to ensure thorough care. Our doctors craft a treatment strategy and rehabilitation plan tailored to each patient’s needs.
Contact us
Get a free online consultation to learn about the expected results of stem cell therapy for your case, what is the cost of the treatment, and its duration.
List of References:
Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet. 2020 Nov 28;396(10264):1711-1712. doi: 10.1016/S0140-6736(20)32230-3. Epub 2020 Nov 4. PMID: 33159851.
Razak HRBA, Corona K, Totlis T, Chan LYT, Salreta JF, Sleiman O, Vasso M, Baums MH. Mesenchymal stem cell implantation provides short-term clinical improvement and satisfactory cartilage restoration in patients with knee osteoarthritis but the evidence is limited: a systematic review performed by the early-osteoarthritis group of ESSKA-European knee associates section. Knee Surg Sports Traumatol Arthrosc. 2023 Dec;31(12):5306-5318. doi: 10.1007/s00167-023-07575-w. Epub 2023 Sep 22. PMID: 37737920; PMC10719133.
Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013 May;21(5):303-11. doi: 10.5435/JAAOS-21-05-303. PMID: 23637149; PMCID: PMC4886741.
Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014 Jul;78(3):188-98. doi: 10.1016/j.maturitas.2014.04.017. Epub 2014 May 2. PMID: 24855933.
Xiang XN, Zhu SY, He HC, Yu X, Xu Y, He CQ. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res Ther. 2022 Jan 10;13(1):14. doi: 10.1186/s13287-021-02689-9. PMID: 35012666; PMCID: PMC8751117.
Le H, Xu W, Zhuang X, Chang F, Wang Y, Ding J. Mesenchymal stem cells for cartilage regeneration. J Tissue Eng. 2020 Aug 26;11:2041731420943839. doi: 10.1177/2041731420943839. PMID: 32922718; PMCID: PMC7457700.
Emadedin M, Labibzadeh N, Liastani MG, Karimi A, Jaroughi N, Bolurieh T, Hosseini SE, Baharvand H, Aghdami N. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018 Oct;20(10):1238-1246. doi: 10.1016/j.jcyt.2018.08.005. Epub 2018 Oct 11. PMID: 30318332.
Sok D, Raval S, McKinney J, Drissi H, Mason A, Mautner K, Kaiser JM, Willett NJ. NSAIDs Reduce Therapeutic Efficacy of Mesenchymal Stromal Cell Therapy in a Rodent Model of Posttraumatic Osteoarthritis. Am J Sports Med. 2022 Apr;50(5):1389-1398. doi: 10.1177/03635465221083610. PMID: 35420503.
Lee JS, Shim DW, Kang KY, Chae DS, Lee WS. Method Categorization of Stem Cell Therapy for Degenerative Osteoarthritis of the Knee: A Review. Int J Mol Sci. 2021 Dec 11;22(24):13323. doi: 10.3390/ijms222413323. PMID: 34948119; PMCID: PMC8704290.
McKay J, Frantzen K, Vercruyssen N, Hafsi K, Opitz T, Davis A, Murrell W. Rehabilitation following regenerative medicine treatment for knee osteoarthritis-current concept review. J Clin Orthop Trauma. 2019 Jan-Feb;10(1):59-66. doi: 10.1016/j.jcot.2018.10.018. Epub 2018 Oct 26. PMID: 30705534; PMCID: PMC6349636.
Padda J, Khalid K, Zubair U, Al Hennawi H, Yadav J, Almanie AH, Mehta KA, Tasnim F, Cooper AC, Jean-Charles G. Stem Cell Therapy and Its Significance in Pain Management. Cureus. 2021 Aug 17;13(8):e17258. doi: 10.7759/cureus.17258. PMID: 34540482; PMCID: PMC8445610.
Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016 Mar;19(3):219-25. doi: 10.1111/1756-185X.12670. Epub 2015 May 20. PMID: 25990685.
Judson RN, Quarta M, Oudhoff MJ, Soliman H, Yi L, Chang CK, Loi G, Vander Werff R, Cait A, Hamer M, Blonigan J, Paine P, Doan LTN, Groppa E, He W, Su L, Zhang RH, Xu P, Eisner C, Low M, Barta I, Lewis CB, Zaph C, Karimi MM, Rando TA, Rossi FM.
T. Mark Campbell, F. Jeffrey Dilworth, David S. Allan, Guy Trudel. The Hunt Is On! In Pursuit of the Ideal Stem Cell Population for Cartilage Regeneratioz Front. Bioeng. Biotechnol., 27 May 2022. Sec. Cell and Gene Therapy. Volume 10 – 2022 | https://doi.org/10.3389/fbioe.2022.866148
Inhibition of Methyltransferase Setd7 Allows the In Vitro Expansion of Myogenic Stem Cells with Improved Therapeutic Potential. Cell Stem Cell. 2018 Feb 1;22(2):177-190.e7. doi: 10.1016/j.stem.2017.12.010. Epub 2018 Jan 25. PMID: 29395054; PMCID: PMC6031334.
MD, Endocrinologist, Pediatrician, regenerative medicine specialist, R&D director






