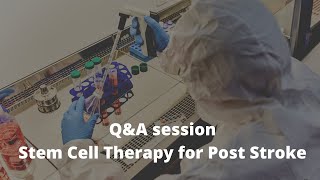
Living with ataxia can gradually reshape daily life—walking may become unsteady and unpredictable, coordination can decline, and everyday tasks that once felt automatic often require greater effort and planning. Ataxia may also affect speech, making it less clear or more difficult to control.
Standard medical care focuses on symptom management and rehabilitation, but many forms of ataxia still have limited disease-modifying options.
For that reason, stem cell therapy for ataxia is being explored as a supportive regenerative approach. The goal is not to replace established neurological care but to complement it, to support functional recovery, and to enhance the effects of ongoing rehabilitation.
What Ataxia Is and How It Affects the Nervous System
Ataxia is a clinical syndrome rather than a single diagnosis. It describes poor muscle control and impaired coordination due to dysfunction in the cerebellum, its connections, or sensory pathways that help the brain interpret body position.
Causes include inherited disorders, stroke, tumors, infections, medication effects, alcohol-related damage, autoimmune and degenerative neurological conditions (such as multiple sclerosis).
Why Coordination and Balance Become Impaired
Coordinated movement relies on continuous feedback: the nervous system compares intended movement with actual movement and corrects errors rapidly. When cerebellar circuits or key sensory inputs are disrupted, timing and precision degrade, leading to unstable gait and reduced fine motor control.
The Role of the Cerebellum and Sensory Pathways
The cerebellum supports timing, posture control, balance, speech articulation, and eye movement coordination. Sensory pathways—especially proprioception from muscles and joints—inform the brain where the body is in space. When these systems fail, stability becomes less automatic and requires more conscious effort.
Difference Between Cerebellar and Sensory Ataxia
Depending on which of these systems is affected, the symptoms manifest differently:
- Cerebellar ataxia: impaired coordination persists even with visual guidance; speech and eye movement changes may occur.
- Sensory ataxia: balance often worsens in darkness or with eyes closed because vision can no longer compensate for impaired proprioception.
Common Symptoms of Ataxia and Disease Progression
Ataxia can progress slowly over years, especially in degenerative or inherited forms, while sudden onset may occur after acute neurological events. Symptom patterns depend on the underlying cause and which networks are affected.
Motor Symptoms and Gait Instability
Impaired posture and gait stability are associated with the discoordination of signals in neural pathways, which in turn coordinates the muscles involved in movement.
Common motor features include:
- wide-based, unsteady gait
- difficulty turning or stopping safely
- frequent stumbles or falls
- reduced hand coordination (writing, buttons, utensils)
Speech and Swallowing Difficulties
Dysarthria (slurred or slowed speech) may develop as coordination of speech muscles becomes less reliable. Swallowing difficulties can occur and may require assessment to reduce choking and aspiration risk.
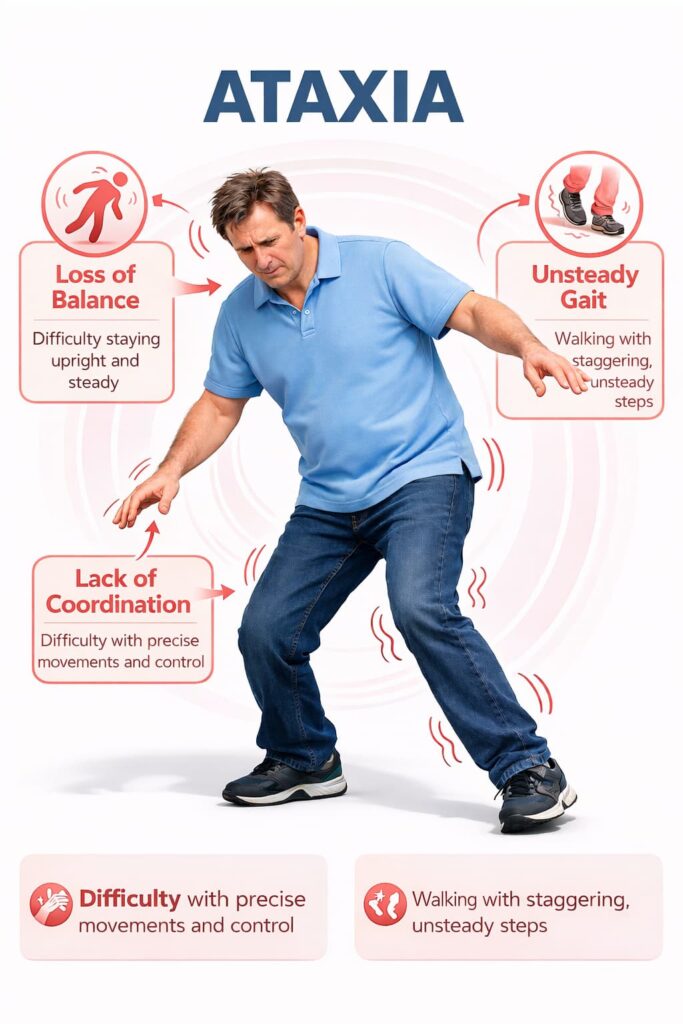
Oculomotor and Visual Coordination Problems
Eye movement control may be affected, leading to nystagmus and visual instability, dizziness, or difficulty tracking objects—symptoms that can increase fatigue and fall risk.
Conventional Treatment Options and Their Limitations
Conventional care remains the foundation of ataxia management. Treatment usually combines symptom control, rehabilitation, and safety planning. For certain etiologies, addressing a reversible cause can be meaningful; however, many forms of ataxia remain chronic and progressive.
Medications and Symptomatic Therapy
Medication strategies depend on the clinical picture and may target tremor, spasticity, vertigo, sleep disruption, mood symptoms, or neuropathic pain. Cause-directed therapy is essential when a treatable driver is identified.
Physiotherapy, Speech Therapy, and Assistive Devices
Rehabilitation is often the most practical way to protect function and reduce complications, yet it is frequently overlooked and rarely receives the clinical attention it deserves. A comprehensive plan may include:
- balance and gait training
- strength and endurance work
- fall prevention strategies and home modifications
- speech and swallowing therapy
- assistive devices (cane, walker, orthotics as needed)
A randomized clinical trial in cerebellar ataxias found that home-based high-intensity aerobic training led to greater improvements in ataxia symptoms than home balance training, supporting structured exercise as a core component of long-term management.
Why Traditional Treatments Cannot Reverse Neural Damage
Rehabilitation improves compensation and function, but many ataxias involve ongoing degeneration or network disruption. Symptom-focused approaches typically cannot restore lost cerebellar neurons or reverse established cerebellar tissue changes.
New Treatments for Ataxia: Why Regenerative Therapies Are Being Explored
Because many forms of ataxia have limited options beyond rehabilitation and symptom management, new treatments for ataxia increasingly are being developed with a growing focus on regenerative strategies. One question often comes up early: what are mesenchymal stem cells (MSCs) in regenerative therapy, and what abilities do they have?
Neuroprotective and Anti-Inflammatory Effects
MSCs are studied for their ability to modulate immune activity and release signaling molecules that may reduce harmful inflammation.
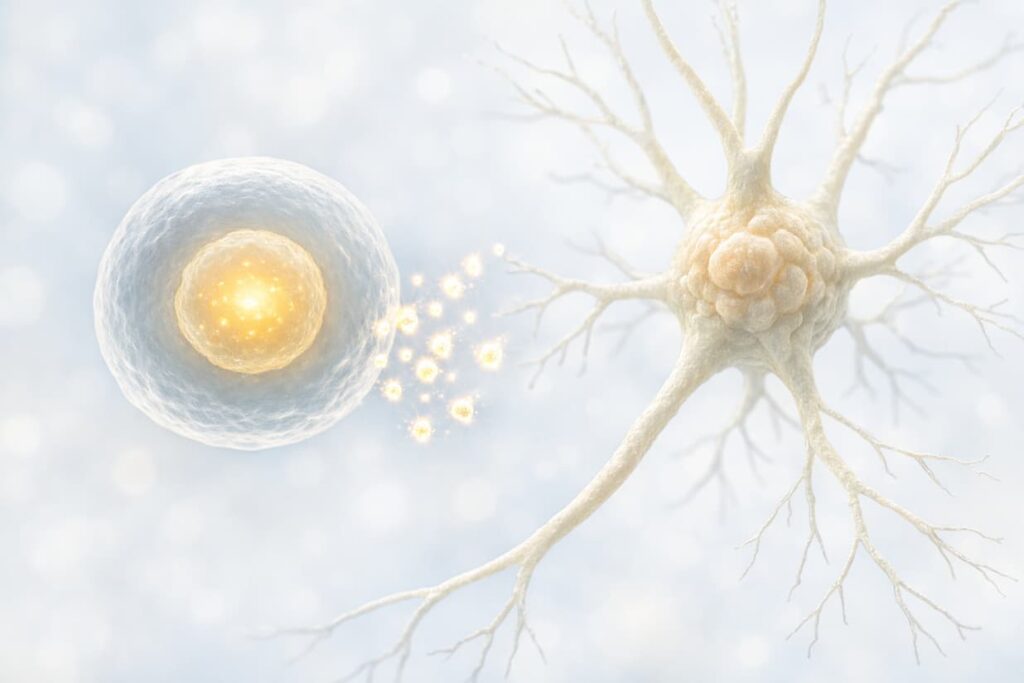
Many proposed effects are “paracrine,” meaning the cells influence the environment through secreted factors rather than replacing large numbers of neurons directly.
Support for Neuronal Signaling and Connectivity
On a practical level, the goal is reducing inflammation in the neural environment and helping the nervous system work in a more stable way.
By removing damaging factors, stem cells work on the microenvironment, creating conditions for the formation of new neural connections. While structural damage cannot always be fully reversed, this stimulation of existing neurons and pathways helps unlock potential functional improvements.
Potential to Slow Functional Decline
In progressive disorders, the most realistic proposed benefit is supporting day-to-day function and possibly slowing functional worsening.
Get a free online consultation
Are you interested in new treatments for ataxia within regenerative medicine? You can book a free online consultation with a qualified regenerative medicine specialist to answer all your questions. Simply fill out the form below.
Can Stem Cells Cure Neurological Diseases Like Ataxia?
At the moment, stem cell therapy cannot cure ataxia. Many forms of ataxia involve long-term changes in cerebellar neurons that current medicine cannot fully reverse.
What Current Research Suggests
Preclinical research is encouraging. One study found that treatment with human MSCs was associated with better motor function and preservation of Purkinje cells—key cerebellar neurons for coordination.
Another study suggested that MSCs may help nerve cells cope better with harmful proteins seen in some genetic ataxias. After MSC exposure, researchers observed signs of stronger cell survival, less cell damage, and healthier neuron structure needed for signal transmission. The authors also reported activation of the cell’s internal “cleanup” pathway, which was associated with a lower level of the toxic mutant protein.
What Improvements Are Realistic
Stem cell therapy is not a cure for ataxia. The goal is to support potential functional improvement, reduce symptom burden, or stabilize disease progression in patients, alongside rehabilitation and standard neurological care. Outcomes vary by diagnosis, disease stage, baseline disability, and rehabilitation consistency.
How Stem Cell Therapy Works in Ataxia
The Swiss Medica program focuses on providing regenerative support for ataxia using MSCs.
These cells may help modulate the tissue microenvironment through neurotrophic signaling and support recovery processes. All stages are coordinated within the clinic’s stem cell injection process.
Types of Stem Cells Used in Our Clinic
We can use two MSC sources, depending on the diagnosis, disease stage, and overall medical profile:
- Autologous MSCs (the patient’s own cells)
These cells are collected from the patient (most often from bone marrow) and prepared within a controlled clinical workflow. This option is considered when an individualized approach is preferred. - Allogeneic MSCs (donor-derived cells)
These MSCs come from placenta and umbilical cord tissue collected from carefully screened donors after healthy childbirth.
This chapter is just a small part of what you can learn about different types of cells and their functions. Dive deeper with our separate article on cells used in therapy.
Read moreHow Cells Interact With Inflammatory and Neurotrophic Pathways
Once inside the body, MSCs may release signaling molecules around themselves that can act on affected tissues through the following mechanisms:
- reduce excessive inflammatory signaling
- protect neurons from ongoing damage
- promote a more balanced immune response
- support neuronal tissue stress-response pathways
- support vascular and metabolic microenvironments
Effects on Cerebellar Circuits and Peripheral Nerves
Stem cell therapy for cerebellar ataxia may affect cerebellar circuits by releasing bioactive signals that help reduce neuroinflammation and support synaptic stability and neuronal survival.
In peripheral nerves, these signals may promote axonal maintenance and myelin support, which can improve nerve conduction and sensory-motor function
What Improvements Some Patients May Experience After Stem Cell Therapy for Ataxia
At Swiss Medica, cerebellar ataxia stem cell treatment is usually offered as an additional option that may complement a patient’s standard care to help achieve better results. When changes occur, they are usually gradual and most noticeable when paired with structured rehabilitation.
Improved Coordination and Gait Stability
Some patients report improved steadiness, longer walking tolerance, and fewer near-falls, particularly when aerobic and balance training continues after the program.
Reduced Tremors and Involuntary Movements
Tremor intensity and involuntary movement patterns may soften in some cases, but response varies by subtype and baseline severity.
Enhanced Speech Clarity and Fine Motor Control
Consistent speech therapy and coordination training can make speech pacing and fine motor tasks more manageable.
However, individual results may vary depending on the type and stage of the disease.
Patient From the Netherlands: Experience with Stem Cells
A 58-year-old patient living in the Netherlands came to Swiss Medica with progressive genetic ataxia and a clear goal: preserve independence and slow functional decline for as long as possible. Balance problems had started to affect his everyday life and confidence—especially in public, where gait instability was often misunderstood.
After completing the stem cell procedure, the patient emphasized what felt most important in the moment: the treatment felt structured, was not painful, and the process was carried out in a controlled clinical setting with ongoing monitoring
“Balance is everything. The goal is to slow progression and stay independent as long as possible—before a wheelchair becomes the only option.”
This perspective reflects how cerebellar ataxia stem cell treatment is most responsibly framed: supportive care with realistic goals.
Patients often find stories reflecting real experiences of stem cell treatment helpful. You can find more than 500 patient reviews relating to various conditions on our official YouTube channel.
Safety and Scientific Evidence of Stem Cell Therapy for Ataxia
Safety depends on cell source, manufacturing standards, screening, and administration setting. Regulatory agencies emphasize that products marketed as “regenerative” may carry real risks when quality controls and oversight are insufficient.
Safety Profile of MSC Therapy
Across regenerative medicine, MSC-based therapies have been studied in multiple areas, where MSCs have been proven to be the safest cells to use with predictable effects.
Swiss Medica Safety Protocols
At our innovations and stem cell center safety is built into the entire care pathway, not limited to the day of administration. A structured program typically includes:
- Medical screening before anything starts
The team reviews diagnosis, medical history, medications, and recent tests to confirm eligibility.
- Controlled cell handling with documented quality checks
Cell preparation follows strict internal protocols in a controlled environment. Each product is checked according to predefined criteria (such as identity and sterility controls) before it is used in a clinical protocol. - Monitored administration and observation afterward
The route and dose are selected based on the clinical profile. During and after administration, the patient is monitored, and the team is ready to respond if any unwanted reactions occur.
Who May Be a Suitable Candidate for Stem Cell Therapy for Ataxia
Candidacy depends on diagnosis, stability, overall health, and realistic functional goals.
Stem cell therapy for ataxia may be considered when:
- the diagnosis is confirmed (including hereditary, sporadic, or adult-onset forms) and the course is relatively stable
- symptoms persist despite rehabilitation and standard neurological care
- the medical profile allows a regenerative protocol safely
- there is enough functional reserve to work toward practical goals (safer walking, fewer near-falls, clearer speech pacing)
- patient seeks a supplemental regenerative approach to accelerate or enhance the effects of their rehabilitation.
Stem cell therapy for adult-onset ataxia may be discussed in the same context—when symptoms are progressing, but the patient still has a rehabilitation window and clear safety goals.
Stem cell therapy may not be appropriate when:
- there is advanced degeneration with minimal rehabilitation potential remains
- an active malignancy is present or suspected
- there is severe medical instability or an uncontrolled systemic infection
- there are life-threatening impairments in swallowing or breathing
Stem Cell Treatment for Ataxia at Swiss Medica
At Swiss Medica, stem cell therapy for ataxia is regarded as a supportive regenerative approach that may be considered following standard treatment, when deemed medically appropriate.
Initial Neurological Assessment
The program typically begins with:
- neurological evaluation and functional scoring
- review of imaging and diagnostic history
- assessment of fall risk, swallowing safety, and rehabilitation potential
Personalized Regenerative Protocol
Stem cell treatments for ataxia are individualized based on diagnosis, severity, and safety profile. The team explains the plan in practical terms: what will be done, why it is recommended, and how progress will be monitored. In most cases, a personalized protocol includes:
- selection of cell source (patient-derived or donor-derived MSCs)
- administration route and schedule chosen for the clinical profile
- on-site monitoring and safety checks
Supportive Rehabilitation and Monitoring
Because ataxia affects balance, rehabilitation is essential for staying safe and reducing the risk of falls. Rehabilitation typically includes physiotherapist-guided balance and gait training, along with practical strategies to reduce fall risk. Depending on individual needs, additional physiotherapeutic interventions may also be used, such as electro- or electromagnetic stimulation, targeted ultrasound therapy, and other supportive techniques.
Explore the full stem cell therapy process step-by-step in our dedicated article.
Learn moreTreatment Timeline and Follow-Up Care
Regenerative therapy for ataxia is a course-based approach, which means that stem cells are administered to a person over a short period of time, rather than every day over a long period of time like medication.
Duration of Stay
Programs for international patients typically plan an on-site stay of 5–9 days, depending on protocol complexity and rehabilitation needs.
When Functional Changes May Be Noticed
Changes are typically gradual, with maximum effects seen within 3-6 months. Progress is often linked to continued training and daily movement strategies.
Why Follow-Up Matters
Follow-up planning often includes:
- comprehensive clinical reassessment after treatment
- a home-based balance and endurance routine
- remote check-ins and progress tracking
- reassessment of assistive devices and fall-risk strategy
Cost of Stem Cell Treatment for Ataxia at Swiss Medica and Worldwide
Patients comparing international options often start with one practical question: what country is best for stem cell treatment when balancing medical standards, total cost, and the level of coordinated care?
The stem cell injection cost varies based on diagnosis complexity, cell source, delivery route, length of stay, and rehabilitation intensity. Our clinic offers more affordable options compared to other popular destinations for regenerative therapy and includes not only a personalized treatment plan but also all other expenses associated with your stay at the clinic.
| Option | Typical Model | Broad Range | Notes |
| Stem cell treatment for ataxia in the UK | Limited private offerings; case-dependent | $10,000–$30,000+ | Availability and indication coverage vary. |
| Stem cell therapy for ataxia in Europe | Varies widely by country and provider | $10,000–$25,000+ | Standards and rehab integration differ across clinics. |
| Stem cell treatment for ataxia in the USA | Often higher costs; trial or private pathways | $15,000–$50,000+ | Regulatory status and product standards require careful verification. |
| Stem cell therapy for ataxia at Swiss Medica–Serbia | Integrated program (assessment + regenerative protocol + monitoring) | €7,000–€31,000* | Often chosen for structured pathways and coordinated follow-up planning. |
*Prices are indicative and based on 2026 estimates; they may vary depending on condition severity and required cell quantity.
Why Patients Choose Swiss Medica
Patients with ataxia have trusted Swiss Medica for our clinical expertise since 2011 and the depth of personalized care we provide at every stage of treatment.
Here’s what sets us apart:
- Personalized management protocols for neurological and neurodegenerative conditions
- A multidisciplinary team (neurology + rehabilitation + nursing monitoring) to support each patient’s symptoms, function, and overall resilience
- Spacious, easy-to-navigate hallways and calm, secure patient rooms designed for comfort, safety, and restorative rest
- Wheelchair-accessible clinical and rehabilitation equipment to support mobility challenges common in ataxia
- Integrated rehabilitation planning and functional follow-up
- Advanced regenerative in-house laboratory aligned with EU GMP Grade A standards for sterile operations and routine sterility testing
- All-inclusive stay for the patient and one accompanying person, airport transfers, meals and accommodation, and interpreter support
- Transparent discussion of the expected cost of stem cell treatment for ataxia and what drives program variability
How to Start a Consultation for Stem Cell Treatment for Ataxia
The patient journey typically begins with a short intake and medical record review.
- Online consultation: medical history, diagnosis details, goals, and current limitations
- Record review: imaging, prior diagnostics, rehabilitation background, and comorbidities
- Eligibility assessment: candidacy, safety considerations, and a realistic plan (including timeline and rehabilitation needs)
Contact us
If you decide to proceed, our team coordinates scheduling, travel logistics, and the individualized treatment plan.
Frequently Asked Questions
1. Which types of ataxia respond best to stem cell therapy?
In practice, stem cell therapy for cerebellar ataxia is discussed more often when the diagnosis is confirmed and the patient still has functional reserve—meaning cerebellar circuits are not in an advanced stage of degeneration and rehabilitation potential remains.
2. How long do potential benefits last after stem cell treatment for ataxia?
Benefits may last from 6 months to a year or longer. Maintenance tends to depend on ongoing rehabilitation, fall risk management, and consistent training strategies.
3. Can stem cell therapy replace physiotherapy?
No. Rehabilitation remains foundational, and functional gains are closely linked to consistent training. However, stem cell therapy can act as an amplifier, enhancing the biological response to exercise and potentially accelerating the progress made during physiotherapy sessions.
4. Can I bring a caregiver?
Yes—you can bring a caregiver, family member, or companion with you to Swiss Medica. One accompanying person’s accommodation and meals are included in the cost of your program.
5. Is stem cell treatment for ataxia available in the UK or Europe?
In practice, stem cell treatment for ataxia in the UK is often discussed in the context of stricter oversight and more limited options outside research settings.
Stem cell therapy for ataxia in Europe can vary widely in how programs are organized—what is included in diagnostics, rehabilitation, monitoring, and follow-up.
List of References:
Dabrowska Sylwia , Andrzejewska Anna , Janowski Miroslaw , Lukomska Barbara. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases, Frontiers in Immunology, 2021. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.591065
Appelt PA, Comella K, de Souza LAPS, Luvizutto GJ. Effect of stem cell treatment on functional recovery of spinocerebellar ataxia: systematic review and meta-analysis. Cerebellum Ataxias. 2021. doi: 10.1186/s40673-021-00130-8.
Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021. doi: 10.1186/s13287-021-02609-x
Zhang X, Liu T, Ran C, Wang W, Piao F, Yang J, Tian S, Li L, Zhao D. Immunoregulatory paracrine effect of mesenchymal stem cells and mechanism in the treatment of osteoarthritis. Front Cell Dev Biol. 2024. doi: 10.3389/fcell.2024.1411507
Chang C, Yan J, Yao Z, Zhang C, Li X, Mao HQ. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv Healthc Mater. 2021 Apr;10(7):e2001689. doi: 10.1002/adhm.202001689. Lee GB, Park SM, Jung UJ, Kim SR. The Potential of Mesenchymal Stem Cells in Treating Spinocerebellar Ataxia: Advances and Future Directions. Biomedicines. 2024. doi: 10.3390/biomedicines12112507
Kim S, Sharma C, Hong J, Kim JH, Nam Y, Kim MS, Lee TY, Kim KS, Suk K, Lee HW, Kim SR. Post-symptomatic administration of hMSCs exerts therapeutic effects in SCA2 mice. Stem Cell Res Ther. 2024. doi: 10.1186/s13287-024-04020-8
Medical Advisor, Swiss Medica doctor