There has been an increase in the development of regenerative medicine recently. One of these new approaches — placental stromal cell therapy, also known as placental stem cell therapy. It uses cells derived from the placenta, the nutrient-rich organ that nourishes a developing fetus.
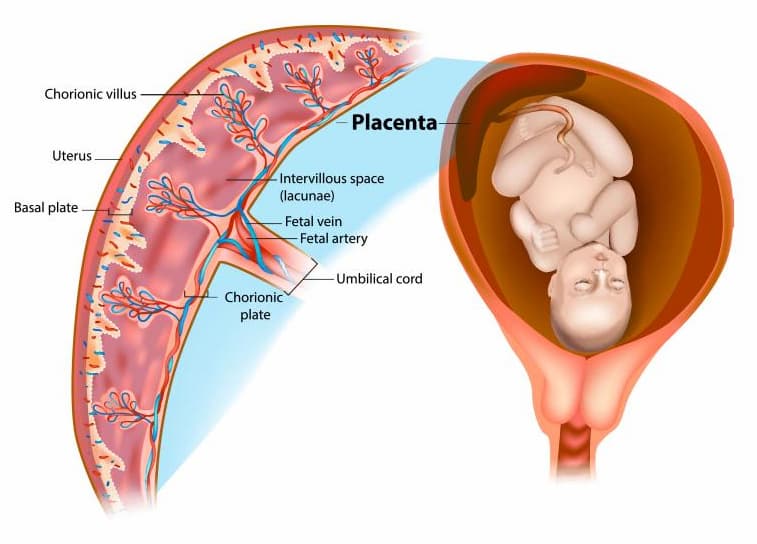
One of the major sources of biomaterial for allogeneic cell products is the human placenta. The placenta is an organ formed in the fetal egg during gestation to ensure the normal development and functioning of the fetus over the intrauterine period. Once the baby is born, the placenta is no longer required, and it may be cryopreserved (preserved for a long time at low temperatures) to treat some conditions and ameliorate someone’s health.
What Is the Advantage of Allogeneic Placenta-Tissue-Derived Stem Cells Over MMSCs Derived From Bone Marrow or From Adipose Tissue?
There are several placenta stem cells benefits over other sources, such as bone marrow or adipose tissue-derived stem cells:
- Stem cells from the placenta have a higher potential for reduplication than stem cells obtained from other sources.
- If your own MSC cells carry a genetic disorder, then donor cells from the placenta will not carry this defect, due to which the disorder has developed
- The number of MMSCs that can be isolated is also much higher. As a result, they allow for less manipulations so the total time decreases, also reducing all the risks associated with in vitro cultivation.
- MMSCs also have immunomodulatory and immunosuppressive properties, regardless of their source. This is why this type of cell is generally used for the treatment of autoimmune responses. The latest placenta stem cell research established that placenta-derived MMSCs have stronger immunomodulatory properties (based on comparative analysis).
- Another placenta stem cells benefit is the possibility of providing immediate cell therapy for some patients with severe conditions or fast disease progression, which cannot be postponed for the period necessary for the preparation as an autologous product.
Moreover, stem cells from the placenta can be used in other situations where the possibility of bone marrow or adipose tissue invasive retrieval is eliminated because of the following:
- The old age of the patient;
- The existence of contraindications for anesthesia;
- High risk of bleeding;
- Chronic blood-thinning medication usage;
- Presence of a pathological process in the anatomical area arranged for sampling.
Sometimes, it is impossible to harvest the required minimum amount of biomaterial for cell isolation or to obtain a sufficient amount of cells from lipoaspirate. For example, slim patients with little subcutaneous fat or patients who just use lipolytic (fat-reducing) drugs.
Also, patients with hematologic diseases and those who underwent long-term hormonal, immunosuppressive, or antibiotic therapy will not have the option of harvesting bone marrow to prepare cell material because of possible damage to bone marrow cells.
Generally, MMSCs retrieved from healthy individuals have proven to have higher therapeutic potential and ability to be used as allogeneic cells. Meanwhile, stem cells from the placenta are the “youngest” and, therefore, the most potent cells that can be retrieved during the postnatal period of a human.
In terms of the benefits obtained from different sources of stem cells, placenta-derived MMSCs are the most promising type of cells for therapy due to all the qualities above.
Get a free online consultation
Contact us to learn about stem cell therapy options for your case and the expected results.
How Do Placenta Stem Cells Work?
After entering the body, placenta stem cells find the affected tissues and fix them by activating local renewal and regeneration processes. When used as a stem cell-based product, MMSCs can repair damaged tissue through paracrine regulation and directly influence the surrounding cells.
Thus, the positive effect of treatment using these cells occurs without the need for medical drugs. These drugs can cause side effects or lead to contraindications. Additionally, there is no need for surgery that may be inapplicable or unwanted due to the patient’s age, condition, or other rational reasons.
Stem cells from the placenta have the ability to promote healing and repair in damaged tissues, as well as aid in restoring dead cells.
What Diseases Can Be Treated with Placental Stem Cells?
Universal therapeutic properties of placental stem cells allow them to be used for treatment and recovery related to many diseases associated with tissue damage, such as
- Digestive system disorders
- Endocrinological (esp. diabetes)
- Rheumatic and heart diseases
- Asthma
- Allergies
- A large number of neurological conditions, such as autism, cerebral palsy, dementia, inclusion body myositis, encephalopathy, multiple sclerosis, Alzheimer’s disease, neuropathy, and others.
Stem cell products obtained from the placenta can be effectively used in programs of total rejuvenation and general health recovery.
How is the Procedure of the Placental Stem Cell Treatment Carried Out?
Once we receive the cells, the laboratory tests them for
- Viral infections;
- Sterility and endotoxins in cell cultures;
- Cell DNA and morphology.
The cells are thawed and cultivated to reach the therapeutic dose as needed. After the placenta stem cell product is prepared, it is transplanted to the patient intravenously, or directly into the affected area.
Ready-made cell products based on placenta stem cell treatment are obtained by a non-invasive method and can be utilized immediately by medical indications. For the patient, there is no need for a long and painful procedure to harvest a portion of their own cells for cultivation.
The recovery period after the placenta stem cell therapy introduction is minimal, but will depend on your situation. In most cases, after the treatment, the patient immediately goes back to their normal way of life.
How Safe is it to Use MMSCs from a Placenta? Risks and Side Effects
Placenta stem cell therapy meets all criteria for multipotent mesenchymal stromal cells based on the newest requirements given by The International Society for Cell Therapy.
MMSCs are also an immune-privileged type of cell. This means that they can stay more or less “invisible” to the immune system, and they are highly biocompatible even with allogeneic material without risk of rejection.
In rare cases, some patient’s bodies will react to the therapy, which usually results in a fever. Either during or after the placental stem cell therapy, the fever passes on its own without consequences to the patient.
For the safety reasons, before use, biological cell material is checked for compliance with several indicators, including:
- A sufficient percentage of viable cells;
- The purity against DNA viruses;
- Sterility against bacteria and microscopic fungi.
Ethical Questions About Using Placenta Stromal Cells: Who is the Donor?
Placental tissue is typically sourced from healthy donors, while stem cells can also be the patient’s own. Many women, during pregnancy, voluntarily and consciously make the choice to donate their placenta for stem cell research after giving birth. They sign an Informed Consent form that prescribes the use of the placenta for scientific purposes and does not exclude its use to assist patients with various diseases. Thus, the placenta provides medical benefits beyond its original function.
Placental mesenchymal stem cells are the best alternative to embryonic stem cells, as the use of the latter is concerned with bioethical issues. Placental stem cells are not obtained during pregnancy and are not related to its interruption. Sampling is performed after the birth of a child when the organ has already completed its functions. Otherwise, it would be disposed of as biomedical waste. Women don’t get money for donating their placenta used for stem cell research, meaning there isn’t a black market for placentas.
Thus, sampling placenta used for stem cells are absolutely harmless and safe for the donor. It is also fully legal, acknowledging it is practiced and conforms to the legislation in the countries. When using the patient’s own cells from the placenta, there are no ethical concerns.

How to Get Treatment Based on Placental Stem Cells?
If you are interested in placental stem cell therapy, you can initiate the process by consulting Swiss Medica’s medical advisor. Placenta stem cell treatment is only part of the complex therapy that we perform.
According to the patient’s current medical needs, placenta stem cell treatment may include preparatory procedures and post-treatment stages of rehabilitation, such as physiotherapy, kinesiotherapy, IMR, and plasmapheresis.
Treatment Program with Placental Stromal Cells at Swiss Medica
At Swiss Medica, we create a personal treatment program according to the patient’s needs to achieve maximum results. Depending on the disease and its severity, treatment can lead to remission, stopping all progress of the disease, or steady improvement.
Before making a decision, first get an online consultation from our Medical Advisor to learn whether and which therapy will work for your condition and get full information about our programs.
We do not prescribe placental stem cell therapy to every patient. We can only advise a reasonable program based on cell products if we assume the possibility of improvement depending on the disease and its features. We are ready to discuss your wishes and expectations about therapy.
Average Cost of Treatment with Placental Stromal Cells
The average cost of treatment with placental stromal cells depends on many factors; the doctor determines the final list of procedures and costs, which range from €7,000 to €25,000. So it’s best to book a consultation with our doctor to find out how much the treatment will cost.
Contact us
Contact us to learn whether stem cell therapy would work for you, and what results are expected.
List of References:
Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, Li X, Yang SG, Han ZB, Han ZC. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016 Nov 10;7(1):163. doi: 10.1186/s13287-016-0418-9. PMID: 27832825; PMCID: PMC5103372.
Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek DV. Placental mesenchymal stem cells as a potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194(3):664-73. https://www.academia.edu/20762939/Placental_mesenchymal_stem_cells_as_potential_autologous_graft_for_pre-_and_perinatal_neuroregeneration
Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta. 2017 Nov;59:87-95. doi: 10.1016/j.placenta.2017.04.003. Epub 2017 Apr 7. PMID: 28411943.
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):11002-7. doi: 10.1073/pnas.0704421104. Epub 2007 Jun 14. PMID: 17569781; PMCID: PMC1891813.
Horwitz E.M., Le Blanc K., Dominici M. et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393-5. https://www.academia.edu/18518567
MD, Pediatrician, Regenerative Medicine Specialist