Recent years have witnessed remarkable progress in the field of stem cell treatment in the USA and around the world. Extensive research has been conducted to assess the effectiveness of stem cell treatment in lowering the incidences of mortality and morbidity associated with several common and rare disorders.
This article discusses the current scenario related to stem cell therapy and research in the US to help you make informed decisions about the different treatment options available for managing your conditions.
Advancements in Stem Cell Treatment in the US
Stem cells can multiply and differentiate into different cell types in the body and rapidly regenerate damaged tissue. Adult multipotent mesenchymal stromal cells (MSCs) are commonly used both in the US and around the world — for instance, in Serbia — for the treatment of degenerative diseases, and certain diseases affecting the skin, bone, and cartilage.
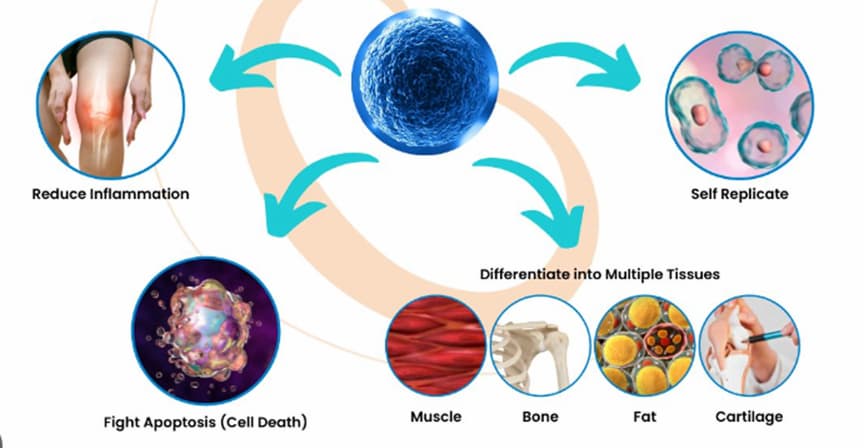
The use of autologous MSCs is regulated by the US FDA (Food and Drug Administration), although not all stem cell treatments have been scientifically proven to be effective.
Get a free online consultation
Please, contact our medical advisor to discuss your health condition with a specialist in regenerative medicine. You can also leave your contact details for a callback. It is free and confidential.
Latest Developments in Stem Cell Therapies in the United States
The results demonstrated by clinical studies and laboratory research aimed at evaluating the prognosis associated with stem cell therapy have been promising.
Some examples of recent developments in stem cell treatment in the USA that can also be applied to the rest of the world include:
Advancements in cell biology
The latest research has provided vital clues about the potential use of exosomes and macrophages in stem cell treatments.
Research has revealed that exosomes can transport active substances such as proteins and RNA, thus improving treatment outcomes. Additionally, unlike living cells, exosomes do not cause an immune response or rejection, suggesting a reduced risk of adverse effects.
Similarly, the use of macrophages in stem cell USA treatments is associated with an improvement in motor and cognitive functions. This is expected to improve the prognosis of the treatment for autism and ischemic stroke. Together, these advancements act as a good basis for stem cell treatments with higher effectiveness – like the one used at Swiss Medica.
Improved methods for expanding stem cells
Researchers have developed more effective methods, such as using a spinner flask bioreactor, for expanding stem cells, including pluripotent stem cells. These cells can differentiate into unique cell forms and have the potential to be employed for a range of therapeutic applications.
Wider scope of stem cell-based therapies
The latest research has paved the way for significant progress in the application of stem cell-based therapies for the treatment of several medical conditions. Some of these include diabetes, heart disease, spinal cord injuries, liver cirrhosis, and neurodegenerative diseases.
These promising advancements in US stem cell therapy are expected to revolutionize the way health conditions and injuries are treated not only in the USA but also around the world.
Various stem cell treatments offered in the US
Stem cell USA therapies and those offered at Swiss Medica are based on the use of adult multipotential mesenchymal stromal cells (MSCs). These cells are called adult cells not because they are retrieved from adults, but rather because they are not taken from embryos. Usually, donor cells from the umbilical cord or placenta are used, in combination with exosomes from these cells.
In some cases, adult multipotential mesenchymal stromal cells from the patient’s own body tissues may be used. They come from adipose tissue and bone marrow, but only if determined more suitable by the regenerative specialist.
Conditions and diseases for which stem cell treatments are available
Conditions and diseases for which the US stem cell and Swiss Medica stem cell treatments are available include:
- Neurodegenerative diseases
- Heart failure
- Multiple sclerosis
- Parkinson’s disease
- Diabetes
- Spinal cord injuries
- HIV
- Autism Spectrum Disorder.
Stem Cell Research Initiatives in the US
Stem Cell USA and global research initiatives are aimed at providing more effective ways for the management of common diseases and lowering the prevalence of mortality and co-morbidities associated with them.
Currently, several research initiatives are in Phase I and II of clinical trials. The future of both international and US stem cell treatments depends on whether these studies can establish the safety and effectiveness of MSCs for the management of diseases.
Future Prospects for Stem Cell Therapy in the US
Besides offering a positive outlook on treatment methods in the USA, stem cell research opens a wide range of opportunities for European clinics. Let’s explore some of them:
Barcoding of stem cells
Researchers are pioneering a groundbreaking barcoding technology in which each stem cell serves as a “fingerprint” with a unique DNA sequence.
This allows each barcode to be passed on to all the progeny of the stem cell. Researchers can then use it to trace the lineage of each cell and study how some conditions, such as leukemia, may arise.
RNA biology
Research is being conducted to assess whether RNA can be employed to improve results in stem cell USA therapy as well as regenerative treatments provided in other parts of the world.
Sometimes, methyl and other chemicals get attached to the surface of the RNAs. This allows them to boost protein production. Researchers are studying whether these modified RNAs could help guide treatment development.
Finding Stem Cell Treatment Options in the US
Patients looking for stem cell treatment in the USA have the option to consult specialists in their own country or choose experienced doctors at Swiss Medica. The EU regulations require that cell-based therapies meet good manufacturing practice (GMP) standards. These standards are globally recognized for ensuring the quality control and production of medicines.
You can access information and get answers to any of your questions by talking to one of our specialists. They will offer comprehensive guidance on the cost of stem cell therapy and assess its suitability for your specific condition.
Contact us
Get a free online consultation to learn about the expected results of stem cell therapy for your case, what is the cost of the treatment, and its duration.
List of References:
Collino, F., Pomatto, M., Bruno, S., Lindoso, R. S., Tapparo, M., Sicheng, W., Quesenberry, P., & Camussi, G. (2017). Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem cell reviews and reports, 13(2), 226–243. https://doi.org/10.1007/s12015-016-9713-1
Chernykh, E. R., Shevela, E. Y., Starostina, N. M., Morozov, S. A., Davydova, M. N., Menyaeva, E. V., & Ostanin, A. A. (2016). Safety and Therapeutic Potential of M2 Macrophages in Stroke Treatment. Cell transplantation, 25(8), 1461–1471. https://doi.org/10.3727/096368915X690279
Tsai, A. C., & Ma, T. (2016). Expansion of Human Mesenchymal Stem Cells in a Microcarrier Bioreactor. Methods in molecular biology (Clifton, N.J.), 1502, 77–86. https://doi.org/10.1007/7651_2016_338
Huang, L., Fu, C., Xiong, F., He, C., & Wei, Q. (2021). Stem Cell Therapy for Spinal Cord Injury. Cell transplantation, 30, 963689721989266. https://doi.org/10.1177/0963689721989266
Hoang, D. M., Pham, P. T., Bach, T. Q., Ngo, A. T. L., Nguyen, Q. T., Phan, T. T. K., Nguyen, G. H., Le, P. T. T., Hoang, V. T., Forsyth, N. R., Heke, M., & Nguyen, L. T. (2022). Stem cell-based therapy for human diseases. Signal transduction and targeted therapy, 7(1), 272. https://doi.org/10.1038/s41392-022-01134-4
Feng, J., Jang, G., Esteva, E., Adams, N. M., Jin, H., & Reizis, B. (2024). Clonal barcoding of endogenous adult hematopoietic stem cells reveals a spectrum of lineage contributions. Proceedings of the National Academy of Sciences of the United States of America, 121(4), e2317929121. https://doi.org/10.1073/pnas.2317929121
Cui, Q., Shi, H., Ye, P., Li, L., Qu, Q., Sun, G., Sun, G., Lu, Z., Huang, Y., Yang, C. G., Riggs, A. D., He, C., & Shi, Y. (2017). m6A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell reports, 18(11), 2622–2634. https://doi.org/10.1016/j.celrep.2017.02.059
MD, Pediatrician, Regenerative Medicine Specialist