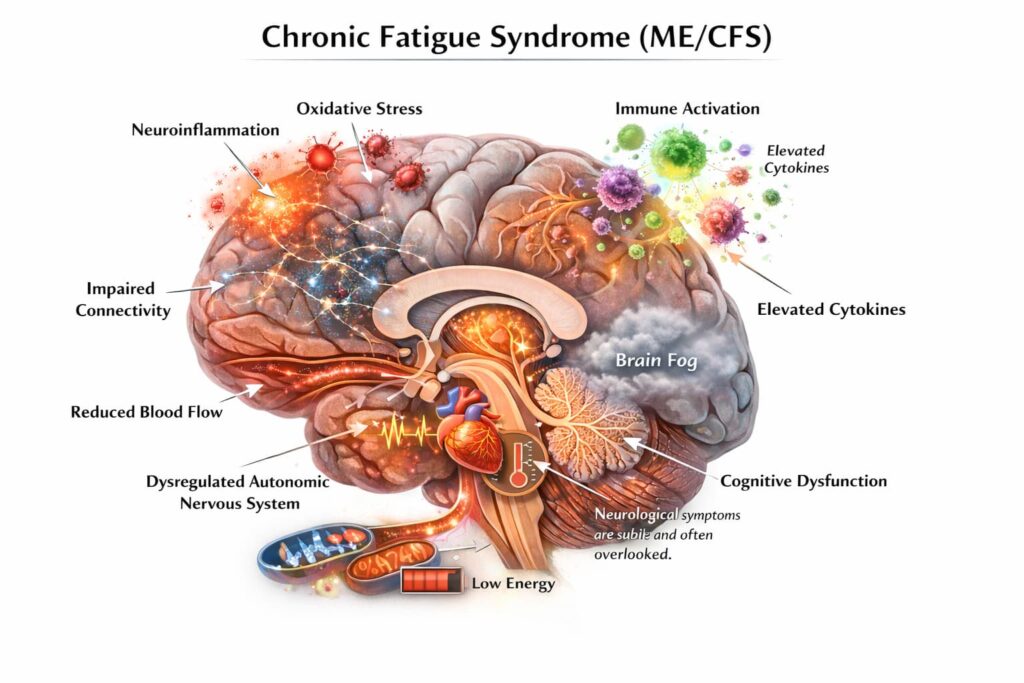
Chronic fatigue syndrome (CFS), also known as myalgic encephalomyelitis (ME), is a condition marked by persistent, severe fatigue that cannot be explained by other medical causes like anemia, hormonal imbalances, or depression. It does not improve with rest, and it is accompanied by worsening symptoms after physical or mental exertion, cognitive difficulties, and systemic imbalance. The exact cause remains unclear, and no curative treatment currently exists.
Within this context, stem cell-based therapy is being explored as a new treatment for chronic fatigue syndrome, designed to support the body’s natural repair and regulatory processes rather than act as a direct cure. In this article, we explain how this new treatment for CFS works and which patients may benefit most.
What Is Myalgic Encephalomyelitis?
Before discussing regenerative medicine, it’s important to understand the condition itself and how chronic fatigue affects the body.
ME and Chronic Fatigue Syndrome: What The Terms Mean
The terms “myalgic encephalomyelitis” (ME) and “chronic fatigue syndrome” (CFS) refer to the same medical condition:
- Myalgic encephalomyelitis is thought to be a multifactorial condition influenced by a combination of genetic and environmental factors, like infections or toxin exposure, and other factors.
- Chronic fatigue syndrome highlights ongoing, disabling fatigue as a central symptom.
Many patients and clinicians prefer the term ME/CFS because it emphasizes the severity of the condition and goes beyond the concept of ordinary tiredness.
Why ME/CFS Is More Than “Just Fatigue” And Why It Is Misunderstood
Fatigue is often blamed on long-term stress or overwork, and many people expect it to improve once life becomes calmer. In some cases, a prolonged stressful period or an illness may precede the onset of chronic fatigue syndrome—but stress may act as an initial factor rather than the cause of ongoing symptoms. Once CFS develops, the exhaustion can persist without a clear ongoing reason, does not improve with sleep, and may gradually limit everyday life.
Despite its impact, ME/CFS is still often misunderstood, largely because its symptoms are not visible. Many patients appear outwardly well, and routine medical tests may show no clear abnormalities. Fatigue can become so constant that it is considered “normal” until more obvious changes emerge, like loss of tolerance for everyday activities or noticeable cognitive and nervous system difficulties.
ME/CFS might begin after a viral infection or other immune stress.
- Post-viral causes. Illnesses like COVID-19 have been linked to the onset of persistent fatigue and cognitive symptoms in some patients. Current research suggests that an infection may trigger ongoing immune activation, leading to persistent inflammation, immune imbalance, and oxidative stress that do not fully resolve.
- Immune causes. Research has found changes in immune-cell function and ongoing low-level inflammation in the nervous system of patients with ME/CFS.
The exact causes of ME/CFS are still unknown. Some changes in the immune system have been observed, but it is not yet clear how they relate to the disease or affect symptoms.
Key Symptoms of Chronic Fatigue Syndrome
Patients with ME/CFS experience a constellation of symptoms affecting multiple systems:
- Persistent fatigue. A crushing lack of energy that is unrelieved by rest and lasts for 6 months or longer.
- Post-exertional malaise (PEM). Minor physical, mental, or emotional effort leads to worsening symptoms like deeper exhaustion or poorer sleep.
- Cognitive dysfunction. Difficulties with memory, concentration, information processing, and word-finding.
- Autonomic nervous system imbalance. Many patients with CFS have difficulties with the body’s automatic functions, like regulating heart rate, blood pressure, and digestion.
ME/CFS can be difficult to recognize. Some people have no obvious physical signs despite profound fatigue, which can lead others to misunderstand or underestimate the severity of the symptoms. Others may experience flu-like symptoms like a low-grade fever, a sore throat without an active infection, or tender lymph nodes.
Current Treatment Approaches and Their Limitations
Managing ME/CFS is challenging, and no single standard treatment exists. Current approaches primarily focus on symptom relief.
| Approach | What It Involves | Limitations |
| Symptom management and pacing | Balancing activity and rest to reduce flare-ups and avoid post-exertional crashes, often adjusted based on co-existing conditions associated with CFS. | Helps prevent worsening but does not eliminate fatigue; daily activity often remains limited. |
| Medications and supportive care | Medications may be used to manage pain, sleep problems, anxiety, or other symptoms; some patients may benefit from alternative treatment for chronic fatigue, like meditation, gentle acupuncture, or supplements. | No medication is approved specifically for ME/CFS; benefits are often partial and vary between individuals. |
Why No Single Standard Treatment Exists
CFS affects people differently, with varying triggers and biological changes, making a universal treatment unlikely. New treatments for CFS are currently being investigated, including immunomodulators and regenerative therapies that may provide additional support alongside standard care.
Why Regenerative Therapies Are Being Explored for ME/CFS
Given the possible role of immune system dysfunction in ME/CFS, researchers have turned attention to regenerative medicine as an alternative treatment for chronic fatigue. Mesenchymal stem cells (MSCs) are of interest because they may help create a more supportive internal environment for recovery through the following mechanisms.
Immune Modulation and Inflammation Control
MSCs interact with immune cells and release signals that help reduce excessive inflammation. This may be relevant for ME/CFS, where ongoing immune activation and chronic inflammation are thought to contribute to symptoms.
Support for Nervous System Function
MSCs release factors that support nerve cells and blood flow in the brain. This may help with neurological symptoms like brain fog, dizziness, and sensory sensitivity. Rather than repairing damage directly, stem cells for chronic fatigue may create conditions that support nervous system recovery.
Cellular Energy and Mitochondrial Support
MSCs may influence how cells regulate energy production and reduce oxidative stress. This is relevant for ME/CFS, where impaired energy metabolism is commonly reported.
Get a free online consultation
Results with stem cell treatment for myalgic encephalomyelitis may differ between individuals. Swiss Medica offers a free, no-obligation consultation where our doctors review your case and provide a clear overview of whether this approach may be suitable for you and what outcomes can be expected.

Medical Advisor, Swiss Medica doctor
How Stem Cell Therapy Works in Chronic Fatigue Syndrome
Now, we’ll take a closer at how mesenchymal stem cells for chronic fatigue are used and how they may act in the body.
Types of Stem Cells Used in Stem Cell Therapy for Chronic Fatigue
In regenerative therapies, MSCs are used because of their established safety profile and ability to regulate immune activity. They are classified as adult stem cells to distinguish them from embryonic stem cells.
MSCs may be sourced in two ways:
- Patient’s own cells (autologous MSCs): collected from tissues like bone marrow or adipose tissue.
- Donor cells (allogeneic MSCs): ethically sourced from the placenta and umbilical cord after healthy births.
Treatment is minimally invasive and usually delivered through IV infusions.
Learn more about the different types of stem cells used in regenerative medicine. In our dedicated article, we explain where donor cells come from and how adult stem cells differ from other cell types.
Read moreStem Cells for Chronic Fatigue: How They Act
After administration, MSCs act through a series of steps:
- Enter the bloodstream and move to damaged areas of the body.
- Respond to chemical signals released from areas of inflammation or immune imbalance.
- Release signaling molecules that help regulate immune activity and reduce inflammation. These signals support tissue repair and help restore balance in affected systems.
For people with ME/CFS, this may mean better support for the nervous system and improved energy regulation on a cellular level. However, mesenchymal stem cell treatment is considered supportive, not curative, and is intended to complement existing care.
Learn more about how MSC therapy for chronic fatigue syndrome and other complex conditions works in our detailed article.
Read moreSafety of Stem Cell Therapy for Chronic Fatigue
When provided in a regulated medical setting, MSC therapy for chronic fatigue and other conditions has shown a generally high safety profile in clinical studies:
- Immune tolerance: MSCs rarely trigger immune reactions, which is why they are usually well tolerated by patients.
- No tumor risk: Current evidence does not indicate an increased risk of tumor formation associated with MSC therapy.
At Swiss Medica, a stem cell hospital in Serbia, safety is managed at every stage of treatment. This is how we choose, control, monitor, and work with stem cells for chronic fatigue:
- Each patient undergoes careful medical screening.
- Treatment is delivered under continuous clinical supervision.
- All procedures and stem cell production follow European medical standards.
We processed stem cells in our own GMP-certified laboratory using strict sterility, quality, and safety checks to ensure consistency and minimize risk throughout the entire treatment process.
Possible Side Effects of Stem Cell Treatment for Myalgic Encephalomyelitis
Some patients may wonder, “Are stem cell injections safe?” In our clinical practice and global studies, the reported side effects are typically mild and short-term and may include:
- Temporary fatigue or flu-like sensations
- Headache or low-grade fever
- Mild discomfort at the injection or infusion site
What Improvements Patients May Experience after Stem Cell Treatment for Chronic Fatigue Syndrome
According to studies and feedback from our patients, many people experience improvements in a few key areas following stem cell therapy for CFS:
| Area of Improvement After Stem Cell Transplant for CFS | What Was Observed |
| Energy regulation and stamina | Reduced fatigue and improved ability to manage daily activities; some participants were able to return to part-time work or maintain a steadier routine. |
| Post-exertional malaise (PEM) | PEM was not eliminated, but episodes were reported as less severe, with better tolerance to physical and mental effort. |
| Cognitive clarity and focus | Improved concentration, memory, and mental clarity, with fewer day-to-day issues related to brain fog. |
| Autonomic balance and stress tolerance | Greater overall stability, including better tolerance to physical and emotional stress and fewer symptom fluctuations. |
— Our patient from Scotland shared his increase in energy level:
“Before treatment, even daily life was exhausting, and I had very little stamina. Now I have noticeably more energy, I can do more throughout the day, and I feel closer to a normal rhythm of life again—something that has made a real difference for me.”
More reviews you can find on our YouTube channel.
Note: Individual results vary.
Who May Benefit Most From Stem Cell Therapy for Chronic Fatigue Syndrome?
Stem cell therapy for chronic fatigue is typically considered based on patient’s diagnosis, symptom pattern, and overall health.
| Patient Group | Why It May (or May Not) Be Appropriate |
| Patients who meet criteria for ME/CFS and experience characteristic symptoms of the condition | The core symptoms of chronic fatigue syndrome are persistent fatigue and post-exertional malaise that are not explained by other medical conditions. |
| Post-viral onset cases | Symptoms that began after infections like flu or COVID-19 may involve immune and inflammatory mechanisms that MSCs are designed to support. |
| Persistent moderate-to-severe symptoms | Patients who remain significantly limited despite standard supportive treatments. |
| Overall stable health | No active infections or acute diseases. |
| May not be suitable | Patients with active cancer, serious blood or clotting disorders, pregnancy, recent major cardiac events, or acute medical instability. |
The Swiss Medica Personalized Program for Stem Cell Treatment for ME/CFS
Swiss Medica provides a personalized program for patients with ME/CFS, offering structured support throughout treatment.
Step 1—Comprehensive Diagnostics and Case Review
The therapy begins with a detailed medical review—our doctors evaluate medical history, current symptoms, prior treatments, and existing diagnoses to confirm ME/CFS and identify contributing factors or comorbidities. Based on this, we can understand outcomes and your suitability for the treatment.
Step 2—Individualized Regenerative Protocol
If you are a candidate, we offer a personalized MSC-based protocol. Cell type and dose, delivery method, and treatment schedule are adjusted to your condition.
Step 3—Supportive and Rehabilitative Therapies
We supplement MSC therapy with complementary treatments like physiotherapy, medical device therapies, and Intracellular Metabolism Recovery (IMR) procedures. Together, these approaches help stabilize the body’s condition and support lasting improvements.
Discover what medical device therapies we offer at Swiss Medica and how they can help with specific conditions.
Learn moreTreatment Timeline and Follow-Up
We’ve designed the treatment timeline to help you feel comfortable, supported, and never rushed—and once you leave the clinic, we don’t simply discharge you and move on. We stay in touch before, during, and after the treatment to monitor your progress.
- Clinic stay: most patients stay 3–9 days for treatment, with time to rest and recover in our comfortable, hotel-like hospital in Serbia.
- Recovery pace: if changes occur, they are usually noticed between 1 and 6 months, with pacing and rest remaining essential.
- Ongoing follow-up: regular online check-ins help track progress, adjust guidance, and plan next steps if needed.
Why Patients Choose Swiss Medica
Patients choose Swiss Medica to get stem cell therapy for CFS for the following reasons:
- Expertise in neuroimmune conditions. Our team works specifically with neuroimmune and post-viral disorders like ME/CFS, fibromyalgia, multiple sclerosis, etc.
- Individualized protocols. Treatment plans are adjusted to each patient’s history, comorbidities, and current condition.
- In-house GMP lab. We provide complete control over stem cell quality and safety in accordance with European standards.
- Support for international patients. We have more than 14 years of experience caring for patients from abroad. Our newly constructed, fully equipped hospital provides comfortable accommodations, airport transfers, translation services, and nutritious meals.
- Multidisciplinary medical support. Care is provided by a team that may include neurologists, cardiologists, and rehabilitation specialists.
- Patient-first approach. We assess eligibility carefully and do not recommend stem cell therapy when it is unlikely to be appropriate. Our focus is on realistic expectations and long-term patient trust.
Swiss Medica Hospital spans 10,000 m² and is designed to help patients feel safe and cared for, with 24/7 medical support, nourishing meals, comfortable spaces for rest and walking, and fully accessible rooms and facilities for those with limited mobility.
Treatment Cost
The cost of stem cell therapy varies based on program length, type and number of infusions, and additional services. Typical price ranges include:
| Region | Estimated Cost |
| USA | $15,000–$40,000+ |
| Europe | €12,000–€35,000 |
| Serbia (Swiss Medica) | €7,000–€31,000* |
*Prices are indicative and based on 2026 estimates; they may vary depending on condition severity and required cell quantity.
In the USA and Europe, access is often limited to clinical trials and higher costs, leading some patients to consider stem cell tourism in more accessible locations. In this context, clinic selection and medical standards matter. For example, Swiss Medica follows European-level protocols while offering more affordable options.
How to Start a Consultation
If you’re considering stem cell treatment for ME/CFS and want to explore whether it’s right for you, getting started is simple.
Contact us
Book a free online consultation now—simply leave your contact details in the form. You’ll speak with our doctors about your symptoms, medical history, and concerns, with no obligation to proceed.

Medical Advisor, Swiss Medica doctor
Frequently Asked Questions
1. Can stem cell therapy cure chronic fatigue syndrome?
No, stem cell therapy does not cure ME/CFS but may support symptom improvement by addressing immune and regenerative dysfunctions in some patients.
2. Is stem cell therapy for chronic fatigue a replacement for pacing and symptom management?
No, stem cell therapy complements—not replaces—pacing, medications, and individualized symptom management.
3. How long do potential benefits last?
When benefits occur, they may last from 6 months to 1 year, but duration varies individually.
4. Can I bring a caregiver with me during the stem cell transplant for CFS?
Yes, patients are welcome to bring a caregiver or companion for support during treatment and recovery.
List of References:
Mantle, D.; Domingo, J.C.; Golomb, B.A.; Castro-Marrero, J. Gulf War Illness, Fibromyalgia, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Long COVID Overlap in Common Symptoms and Underlying Biological Mechanisms: Implications for Future Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 9044. https://doi.org/10.3390/ijms26189044
Inami, N. Intravenous Administration of Human-Derived Mesenchymal Stem Cell-Conditioned Medium for Patients with General Malaise. J. Clin. Med. 2025, 14, 5884. https://doi.org/10.3390/jcm14165884
Pada Vinski, D. S. P. V., Trofimova, S., Quintosa, J. R., Nugroho, A. K., Schroeter, C., & Jovanovic, S. (2024). Stem Cell Therapy for Chronic Fatigue Syndrome: A Promising Approach. Journal of World Science, 3(11), 1511–1518. https://doi.org/10.58344/jws.v3i11.1231
Wang, Y., Yi, H. & Song, Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther 12, 545 (2021). https://doi.org/10.1186/s13287-021-02
Xuan, X., Tian, C., Zhao, M. et al. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int 21, 595 (2021). https://doi.org/10.1186/s12935-021-02300-4
MD, Pediatrician, Regenerative Medicine Specialist