If you’ve already done “everything right” for Lyme—testing, treatment, and follow-up—why do symptoms sometimes linger anyway? For some people, the story doesn’t end with antibiotics. Instead, a difficult phase may follow—marked by persistent fatigue, cognitive difficulties (“brain fog”), and joint pain that comes and goes. That’s why patients begin searching for new treatments for Lyme disease that focus on recovery—not only on clearing the infection.
Stem cell treatment for Lyme disease is neither an antimicrobial treatment nor a cure. Instead, it’s a supportive regenerative approach being explored to help modulate immune responses and support tissue repair in selected patients.
Understanding Lyme Disease and Persistent Symptoms
Lyme disease is triggered by infection with Borrelia bacteria transmitted by ticks.
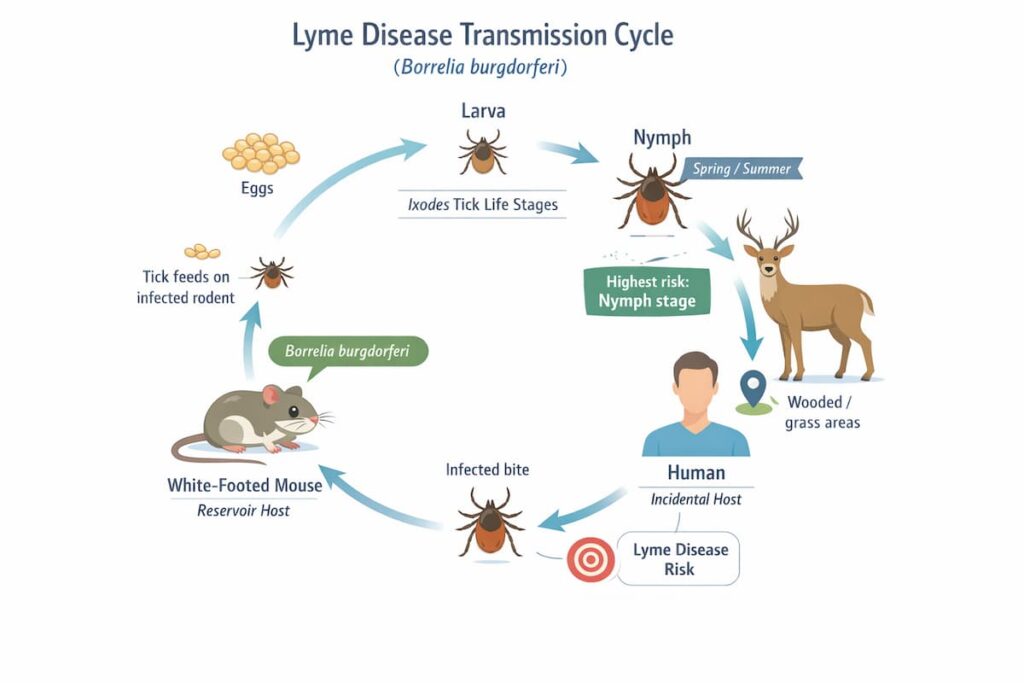
When diagnosed early, standard treatment often works well. But a subset of people report persistent symptoms after finishing therapy—sometimes called post-treatment Lyme disease syndrome (PTLDS) or persistent/post-treatment symptoms, depending on the definition used.
Acute Lyme disease and early treatment
Early Lyme disease may present with a rash (often erythema migrans), fever, headache, fatigue, or muscle and joint aches. When treated promptly with appropriate antibiotics, many patients recover without long-term issues.
Chronic or persistent symptoms after treatment
Persistent symptoms can include fatigue, arthralgias, widespread pain, sleep disruption, mood changes, and cognitive complaints (“brain fog”). Importantly, these symptoms don’t automatically mean there is ongoing active infection—this is one reason the topic can feel confusing and emotionally loaded for patients.
Why inflammation and immune dysregulation may persist
Researchers are exploring multiple, non-exclusive explanations for why symptoms can continue after infection is treated, including:
- ongoing immune activation,
- changes in pain processing,
- metabolic shifts,
- tissue/nerve pathway effects left behind by the earlier inflammatory process.
Standard Lyme Disease Treatments and Their Limitations
Standard care for Lyme disease is evidence-based and remains the first-line approach. However, even good treatment doesn’t always translate into a quick “back to normal” for every patient—especially when symptoms have become chronic or layered with nervous system sensitization.
Antibiotic therapy and supportive care
Antibiotics are used to eliminate the bacterial infection itself, and the choice of regimen depends on clinical presentation:
- early localized disease,
- neurological involvement,
- arthritis, etc.
Supportive care may include pain management, sleep support, graded rehabilitation, and evaluation for coexisting conditions that can worsen fatigue or cognition.
Why some patients continue to have symptoms after treatment
Today, most guidelines recommend careful re-evaluation if symptoms persist rather than automatically extending antibiotics. That’s because lingering fatigue, pain, or cognitive complaints may be driven by ongoing inflammation, nervous system sensitivity, or other factors rather than infection.
This is where additional new treatments for Lyme disease focused on recovery support may be considered—especially when symptom burden remains high despite standard therapy.
Alternative Therapies for Lyme Disease: Why Regenerative Therapies Are Being Explored
When day-to-day life shrinks—work, family, exercise, social plans—patients understandably look for alternative treatments for Lyme disease. This is where regenerative medicine enters the conversation.
Stem cells as a relatively new treatment for Lyme disease are being explored as a natural option for patients with chronic inflammation or immune imbalance that may be contributing to ongoing symptoms.
Can stem cells cure Lyme disease?
Stem cells are not considered a cure, and they are not designed to eradicate Borrelia bacteria.
Alternative treatments for Lyme disease using mesenchymal stromal cells are being explored as supportive care for patients whose main challenge appears to be persistent inflammation, immune dysregulation, or tissue stress after the infection has already been treated. In other words, the focus is on the “aftermath,” not on replacing antibiotics or acting as an antimicrobial therapy.
How does stem cell therapy help Lyme disease?
When we discuss stem cell therapy for Lyme disease, we focus on secondary damage and immune balance—not on killing bacteria. The goal is to support the body’s repair environment when persistent inflammation or immune dysregulation is suspected.
Below are the main supportive targets regenerative medicine teams often discuss.
- Immune modulation and inflammation control
MSCs are widely studied for immunomodulatory and anti-inflammatory signaling. They can influence immune cell behavior through cell-to-cell interactions and, importantly, through the release of bioactive factors (paracrine effects). - Supporting nervous system and tissue recovery
For patients with neurological complaints (cognitive symptoms, neuropathic sensations, autonomic instability), the therapeutic conversation often shifts toward “tissue environment” support—reducing inflammatory signaling and supporting cellular repair pathways rather than targeting microbes. - Addressing secondary damage rather than infection
This is the most important boundary: regenerative approaches are not positioned as antibiotics, and they should not be marketed as antimicrobial solutions.
How Stem Cell Therapy Works in Patients With Persistent Lyme Symptoms
At Swiss Medica, we aim to keep the mechanism discussion clear and grounded: mesenchymal stem cell (MSC) therapy does not “rebuild organs overnight,” and it is not a cure. The interest is in their paracrine signaling—how they may influence inflammation, immune balance, and tissue recovery patterns.
Types of stem cells used (primarily MSCs)
Our stem cell center uses adult multipotent mesenchymal stromal cells (MSCs). Most commonly, these are donor-derived (from ethically sourced placental and umbilical cord tissue). In selected cases, a physician may consider autologous sources (e.g., from bone marrow or adipose tissue) based on individual evaluation.
How stem cells interact with immune and inflammatory pathways
MSCs are often described as “microenvironment modulators.” Instead of directly turning into the exact cells of a damaged tissue, they can release growth factors, chemokines, and other signals that influence the immune activity and the body’s own cells to support repair conditions.
In practical terms, the goal is to help the body transition from a high-alert inflammatory state to a steadier recovery state—when this is clinically appropriate.
Are you interested in learning about stem cells and how they differ? We will help you find out in our article on MSCs.
Learn moreAdministration methods and treatment setting
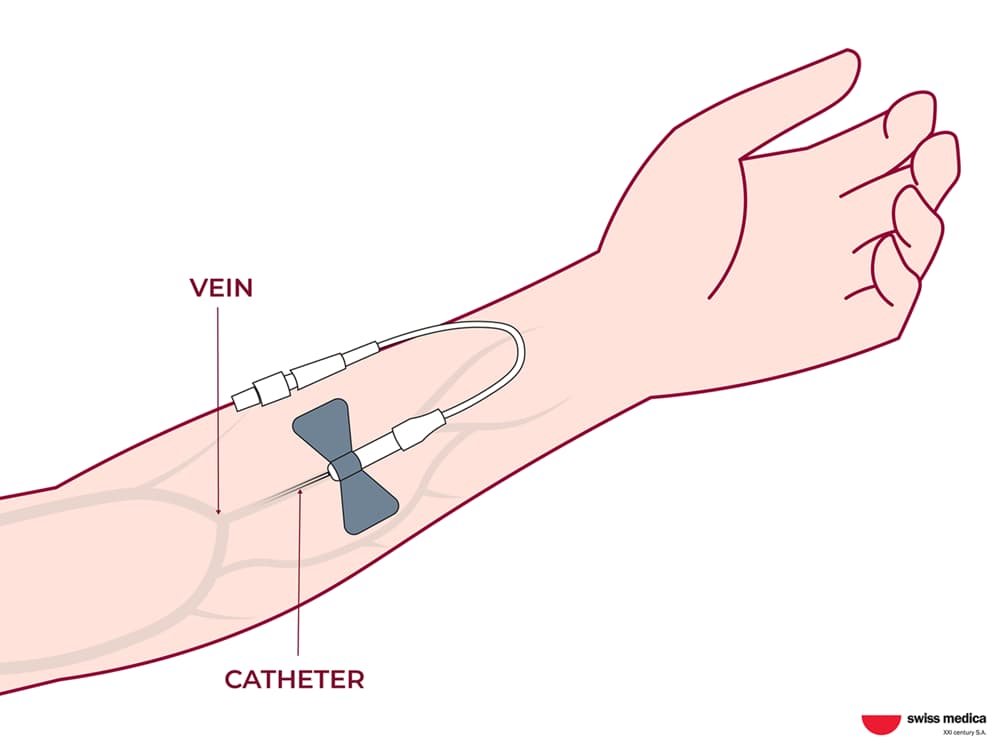
Stem cells release bioactive molecules not only into the microenvironment around them but also affect the entire body as a whole. The method of administration is selected depending on the patient’s complaints and condition.
Common approaches in regenerative medicine include IV administration and local applications:
MSCs may be administered intravenously for systemic support and, in certain cases, delivered through targeted local routes when clinically indicated.
In our programs, clinicians may discuss options such as mesenchymal stem cell injections when explaining how MSCs are administered in a controlled medical setting.
We avoid implying antimicrobial effects at any stage—because that is not what MSCs are designed to do.
Safety and Clinical Evidence for Stem Cell Treatment for Lyme Disease
In this section, we summarize what is known about MSC-based therapy—especially its safety profile—and the current evidence for persistent symptoms after Lyme disease.
Safety profile of MSC therapy
Large reviews and meta-analyses have examined adverse events across many MSC trials and generally describe an acceptable safety profile when therapy is produced and delivered under appropriate standards.
That doesn’t mean “risk-free.” This implies that controlled contexts rarely report complications, underscoring the importance of monitoring protocols.
Want to learn more about how Swiss Medica ensures safe stem cell production? Go to a separate article and dive deeper into the topic.
Read the articleWhat studies suggest about Lyme management
Lyme-specific persistent symptoms are an active research area, and expert discussions highlight immune dysregulation and inflammatory drivers as plausible contributors for some patients.
That’s one reason regenerative approaches are being explored: they align with the hypothesis that, in some patients, the main problem is the aftermath—immune signaling, tissue stress, and nervous system dysregulation—rather than uncontrolled bacterial activity.
Possible Side Effects and Risk Management in Stem Cell Therapy for Lyme Disease
Before discussing possible side effects, it is important to clarify that safety depends not only on the cells themselves but also on clinical standards—patient selection, dosing strategy, sterility controls, and structured monitoring. In reputable medical programs, protocols are individualized to reduce avoidable risks and to ensure early detection of adverse reactions.
At Swiss Medica, the protocol includes laboratory quality control and careful dosing. Cell products go through sterility testing and standard safety checks before use. The dose and route of administration are selected by the clinical team based on the patient’s medical history, current condition, and overall tolerance.
Common short-term effects following MSC-based procedures may include:
- Mild immune reactions (e.g., transient fever or flu-like feelings)
- Fatigue for 24–48 hours
- Temporary discomfort related to the procedure itself
At Swiss Medica, risk management is integrated into the program: patients are monitored during and after administration, vital signs are tracked, and supportive care is adjusted promptly if any reactions occur.
Potential Benefits Patients May Experience
Any potential benefits should be understood as clinical possibilities rather than guarantees. In the context of applying stem cells for Lyme disease, the intended role of the therapy is supportive: helping modulate inflammatory signaling and creating conditions that may favor tissue recovery, with the broader goal of improving day-to-day function.
The areas below reflect symptoms some patients report improving and outcomes clinicians may monitor—depending on the individual’s baseline status, symptom duration, comorbidities, and overall recovery capacity.
Reduced chronic inflammation, pain, and fatigue
Some patients describe a gradual decrease in “whole-body inflammation” sensations—less aching and post-exertional crash, and more stable energy. Changes are usually gradual and can come in waves.
Improved immune balance and stress tolerance
Stem cell therapy for Lyme disease helps reduce chronic inflammation and prevent more tissue damage over time. When the body is less “on high alert,” some people report fewer stress-triggered flare-ups, better day-to-day tolerance, and a more stable baseline overall.
Support for cognitive and neurological symptoms
For brain fog, concentration issues, or neuropathic discomfort, the goal is supportive—to help the body recover after a long inflammatory process. For nerve-related symptoms (tingling, burning, numbness, heaviness), some people report reduced intensity or fewer episodes.
Patient from Australia—real experience
Talia came to Swiss Medica after years of searching for answers and trying different approaches. In her video review, she shares that the regenerative part of the program helped her feel stronger and made everyday life a little more manageable—step by step.
“Stem cells really aided in getting me to where I am today and making me strong. Simple things like having energy to walk down the street or just to get up out of bed—things I couldn’t do last year—really improved.” — Talia S., 22 (Australia)
For a more personal perspective, you can browse 500+ patient stories and video feedback from our patients on our official YouTube channel.
Get a free online consultation
If persistent symptoms after Lyme are still affecting your energy, focus, mobility, or confidence, it may help to get a structured medical review of your case and discuss supportive options.
Book a no-obligation consultation with our regenerative option.
At Swiss Medica stem cell therapy for Lyme disease is considered a supportive regenerative program that may be discussed after standard treatment—when medically appropriate.
Comprehensive diagnostic review
After the initial consultation, we start with a medical record review to understand your history, prior antibiotic treatment, symptom pattern, and risk factors. This step helps our doctors evaluate whether a regenerative approach makes sense—or whether another medical direction should come first.
Personalized regenerative protocol
If you’re eligible, the protocol is individualized: cell source decisions, administration method, and supportive therapies are chosen based on your case rather than a one-size plan.
Supportive therapies
Regenerative therapy is typically positioned as one part of a broader plan. To help patients rebuild function and tolerate gradual increases in activity, we may add supportive physiotherapy and rehabilitation techniques based on symptoms, stamina, and safety considerations.
Depending on your case, the program may include:
- Super Inductive System (SIS) therapy
- IMR therapy
- Combined physiotherapy, which may include:
- Electrotherapy
- Ultrasound therapy
- Low-level laser light therapy (LLLT)
- Spark/Shock wave therapy
Who May Be a Suitable Candidate for Stem Cell Therapy for Lyme Disease
Many candidates share the same pattern: they’ve completed standard therapy, but symptoms remain disruptive enough to justify exploring a supportive regenerative approach.
A patient may be considered for stem cell treatment for chronic Lyme when they have:
- Completed antibiotic treatment under medical supervision
- Persistent inflammatory, pain, fatigue, or neurological symptoms after treatment
- A stable medical situation that allows safe participation in a monitored program
Ineligible patients may include those with:
- Active infection requiring urgent antimicrobial care
- Uncontrolled autoimmune disease activity (case-by-case)
- Malignancy or strong suspicion of malignancy (safety-first approach)
Treatment Timeline and What to Expect
While there is no single schedule that applies to everyone, the outline below reflects the typical structure of a monitored regenerative program and the way outcomes are usually assessed over time.
Length of stay
Programs often require a short in-clinic stay (commonly several days), depending on the protocol and supportive therapies included.
When patients may notice changes
If improvements occur, they may appear gradually—over weeks to 6 months—rather than immediately. Many patients describe subtle early changes (sleep, energy stability) after stem cells for Lyme disease.
Follow-up and monitoring
We prioritize continuity: follow-up recommendations, remote communication, and supportive guidance are part of the patient experience.
Why Patients Choose Swiss Medica
Swiss Medica was founded in 2011 in Switzerland as a group of researchers and clinical physicians in regenerative medicine. In 2016, we relocated our main facilities to Serbia to take advantage of a supportive regulatory environment and convenient logistics for our patients.
Long-standing experience: Since 2011 we’ve helped 10000+ patients with chronic and complex conditions from all over the world.
Multidisciplinary care: Our team works together across specialties (regenerative medicine, rehabilitation, and other medical disciplines as needed) to support each patient’s symptoms, function, and overall resilience.
Own laboratory quality control: We prepare and quality-check cell products under strict protocols, with a controlled lab environment aligned with EU GMP Grade A standards for sterile operations and routine sterility testing.
Affordable, transparent pricing: We offer accessible program pricing—typically from €7,000 to €31,000*—often significantly more affordable than regenerative therapy in Western Europe or the US.
All-inclusive stay: Cost typically includes an all-inclusive stay for the patient and one accompanying person, airport transfers, meals and accommodation, and interpreter support when needed—so you can focus on treatment instead of logistics.
*Prices are indicative and based on 2026 estimates; they may vary depending on condition severity and required cell quantity.
Swiss Medica hospital, which opened in 2024, was built with accessibility and comfort in mind. It boasts spacious hallways, wheelchair-accessible equipment and peaceful, secure patient rooms.
If you’ve been researching the best country for stem cell treatment, we encourage you to look beyond slogans and compare what actually matters: medical oversight, product quality control, transparency, and follow-up structure.
How to Start a Consultation
The patient journey starts with a conversation between a regenerative specialist and the patient, which can be scheduled with just a few clicks.
-
1
Online consultation: You share your medical history and goals.
-
2
Record review: Our team evaluates prior diagnostics and treatments.
-
3
Eligibility assessment: We clarify whether a regenerative approach is appropriate and safe—and what a realistic plan would look like.
Contact us
If you decide to proceed with a stem cell transplant for Lyme disease, we’ll help you plan your visit to Swiss Medica and coordinate you through all steps. Just fill out the form below.
Frequently Asked Questions
1. Does stem cell therapy kill Borrelia bacteria?
No. MSC-based therapy is not an antimicrobial approach and should not be presented as one. Its intended role is supportive—focused on immune modulation and tissue-repair signaling rather than directly targeting bacteria.
2. Is stem cell treatment for Lyme disease a replacement for antibiotics?
No. Stem cell therapy for Lyme disease is not a substitute for antibiotics when an active infection is present or suspected. Standard antimicrobial treatment remains first-line, guided by evidence-based recommendations.
3. Who should not consider stem cell therapy for Lyme disease?
In general, we are cautious with patients who have an active infection requiring urgent treatment, uncontrolled immune-mediated disease activity, or malignancy/suspicion of malignancy. Final decisions are individualized after record review and consultation.
4. How long do potential improvements last after a stem cell transplant for Lyme disease?
We avoid guaranteeing timelines because symptom patterns, biology, and recovery environments differ from person to person. In general, some patients report benefits lasting from 6 months up to a year or more, especially with ongoing supportive care and rehabilitation.
5. Can I bring a caregiver?
Yes. Many patients feel calmer and more confident with a caregiver present, especially if fatigue, mobility issues, or anxiety are part of the symptom picture. We’ll help coordinate practical details during planning.
6. What is the cost of stem cell therapy for Lyme disease?
The cost of stem cell therapy for Lyme disease depends on the recommended protocol and supportive therapies included. As a general reference, programs may range approximately from €7,000 to €31,000*. If you want to compare program options, we can walk you through the stem cell treatment cost factors during a consultation.
7. Can I get stem cell therapy for Lyme disease in the USA?
Patients from North America often ask about availability closer to home, including stem cell therapy for Lyme disease in the USA. However, regenerative medicine is constrained by regulatory pathways, and many offerings patients encounter online are not standardized.
List of References:
M E Baarsma, Joppe W Hovius, Persistent Symptoms After Lyme Disease: Clinical Characteristics, Predictors, and Classification, The Journal of Infectious Diseases, Volume 230, Issue Supplement_1, 15 August 2024, Pages S62–S69, https://doi.org/10.1093/infdis/jiae203
Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021 Oct 18;12(1):545. doi: 10.1186/s13287-021-02609-x.
Wester KE, Nwokeabia BC, Hassan R, Dunphy T, Osondu M, Wonders C, Khaja M. What Makes It Tick: Exploring the Mechanisms of Post-treatment Lyme Disease Syndrome. Cureus. 2024 Jul 20;16(7):e64987. doi: 10.7759/cureus.64987. PMID: 39161484; PMCID: PMC11332314.
Teryek, M. (2025, May 1). Evaluation of Human Mesenchymal Stromal Cells (MSC) as an adjuvant therapeutic for chronic Lyme disease [Conference presentation]. Stratford Research Day, Rowan University. 10.31986/issn.2689-0690_rdw.stratford_research_day.
MD, Pediatrician, Regenerative Medicine Specialist