In recent years, stem cell therapy for autism in India has gained popularity. Parents are motivated by reports of kids saying their first words or feeling better following treatment. But with this promise come crucial considerations about safety, pricing, and what families might realistically anticipate.
In this article, we take an honest look at how stem cells are used for autism, address the question “Is stem cell remedy for autism legal in India?”, and explain what families should understand before considering treatment.
Understanding Autism and Why Families Explore Stem Cell Therapy
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition that affects one in every 65 Indian children aged two to nine. Children with ASD frequently struggle with communication and social interactions:
| Communication difficulties | Difficulties understanding language or articulating requirements, limited gestural communication, and delayed speech development. |
| Social interaction challenges | Avoiding eye contact, acting apathetic while playing with others, and discomfort with physical touch. |
| Repetitive behaviors | Engaging in repetitive movements or behavior, such as hand flapping, rocking, or repeating certain expressions. |
| Resistance to change | Changes in routine can cause discomfort or anxiety, resulting in a preference for strict patterns or familiar surroundings. |
| Sensory sensitivities | Overreacting or underreacting to sounds, lights, textures, or smells (e.g., hiding their face when they hear normal sounds or refusing clothing). |
Each child with autism is unique, but the challenges can have a significant impact on both the child and their family’s quality of life.
There is currently no cure for autism. Existing therapies are designed to manage symptoms and support the development of essential skills. That’s why many parents understandably start exploring for other solutions that might help their children make more progress.
In recent years, stem cell transplantation for autism in India and other countries has emerged as one of these options, attracting growing attention alongside established interventions such as ABA or speech therapy.
What Therapies Are Available for Autism Today?
When exploring stem cell therapy for autism in India or outside, it should be seen as one emerging option within a broad range of interventions—not as a cure on its own. Let’s take a quick look at the different types of therapies that are available, from well-known ones to newer ones, to see where it fits in.
Conventional Therapies (ABA, speech therapy, occupational therapy)
Today, autism care is based on a mix of behavioral and developmental therapies.
| Therapy/Support | What It Does | Notes |
| Applied Behavior Analysis (ABA) | Uses structured, positive reinforcement to teach skills and reduce harmful behaviors. | Considered the “gold standard” with decades of research backing its effectiveness. |
| Speech Therapy | Improves communication skills: language, articulation, and understanding. | Especially important for children with delayed or limited speech. |
| Occupational Therapy | Builds daily living skills, improves motor coordination, and manages sensory issues. | Helps children function more independently in everyday settings. |
| Physical Therapy | Supports children with motor coordination or muscle-tone difficulties. | May overlap with occupational therapy for physical development. |
Emerging Approaches
Beyond traditional therapies, many families are looking for new or emerging interventions. Some popular options include:
| Approach | Description |
| Changes to the diet | Gluten-free and casein-free diets are sometimes tried because parents believe certain proteins affect behavior. Supplements (e.g., omega-3, vitamins) are also common. Can help when intolerances or GI issues exist. |
| Hyperbaric Oxygen Therapy (HBOT) | Breathing pure oxygen in a pressurized chamber to increase oxygen delivery and reduce inflammation. A recent large clinical review suggests behavioral improvements, but overall evidence is conflicting and experts remain skeptical. |
| Neurofeedback and alternative therapies | Includes neurofeedback (brainwave training), music therapy, animal-assisted therapy (e.g., horseback riding, service dogs), and acupuncture. May help with anxiety/attention but results are inconsistent; considered complementary. |
| Regenerative medicine (Stem Cells) | Stem cell therapy, particularly mesenchymal stem cells (MSCs), is gaining popularity. MSCs may modulate the immune system, reduce inflammation, and support neural regeneration, potentially alleviating autism-related challenges. |
Stem Cell Therapy Compared to Other Methods
Stem cell therapy may seem new, but it has been around for more than 50 years. It is still considered experimental for autism today, but more and more studies are showing its potential, especially therapies that use MSCs.
Rather than teaching skills directly, stem cells for autism treatment aims to address underlying biological factors in autism, such as inflammation or immune imbalance. Stem cells may help kids with autism feel calmer and more focused, which can also support them in benefiting more from therapies such as ABA, speech, or occupational therapy.
Quick comparison:
- Traditional therapies (ABA, speech, occupational): Build behavioral and developmental skills through structured learning, with strong long-term evidence.
- Stem cell therapy: Works at the biological level—reducing inflammation, supporting neural repair, and improving readiness to learn—potentially creating a stronger foundation for other therapies to succeed.
Let’s take a closer look at how stem cells work for ASD.
Get a free online consultation
Consult our medical advisor to learn more about stem cell therapy for autism and your child’s specific treatment options and potential outcomes. Schedule a free, no-commitment online consultation with our medical team today.

Medical Advisor, Swiss Medica doctor
How Stem Cell Therapy Works for Autism Spectrum Disorders
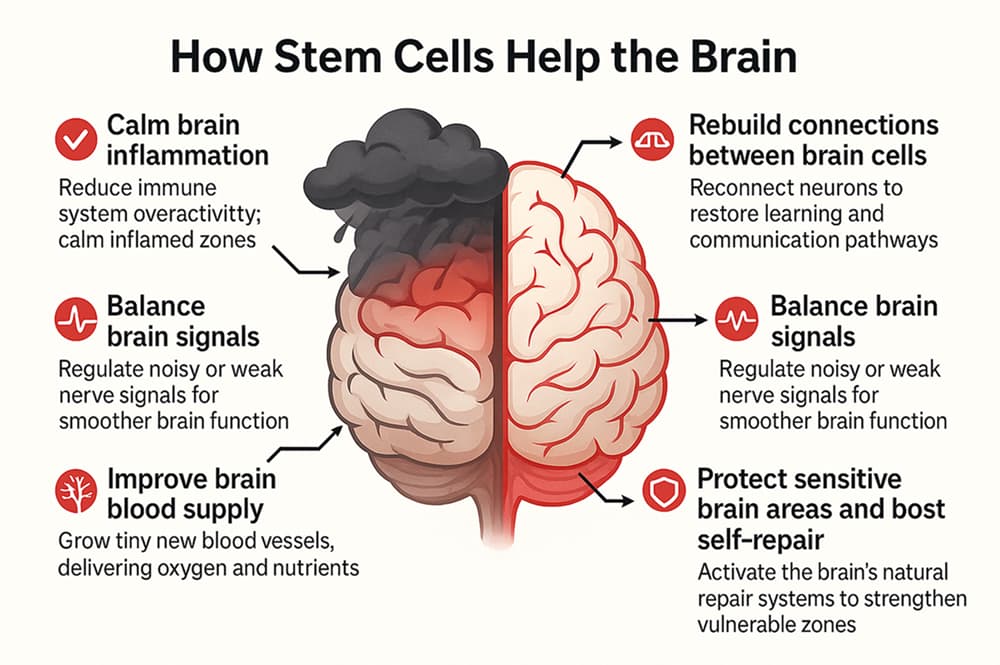
Whether you choose to have a stem cell transplant in India or in another country, the science behind it remains the same, MSCs begin supporting the child’s brain through processes such as:
- Controlling inflammation and modulating the immune system: MSCs release anti-inflammatory factors and work with immune cells to calm an overactive immune system, bringing it back to a healthier state.
- Neuronal support and connectivity: MSCs promote neuroplasticity by stimulating the formation of new synapses, supporting learning and adaptation. They also encourage angiogenesis (new blood vessel growth), which improves cerebral blood flow and addresses reduced perfusion often seen in autism.
- Cytokine regulation: MSCs lower harmful inflammatory cytokines while boosting protective ones, creating conditions for better focus, communication, and overall functioning.
Safety, Risks, and Side Effects
Scientists have been studying stem cell therapy as a possible treatment for the last few decades. Encouragingly, published studies indicate that stem cell treatment for autism in India and other countries with MSCs has a favorable safety profile in children with autism, with most cases reporting only minor side effects like headache or fatigue:
- Low-grade fever for a few hours after the infusion/injection.
- Headache or nausea.
- Local discomfort or pain at the injection site.
- Fatigue for a day or two.
These side effects usually resolve on their own or with minimal medical intervention.
Evidence and Success Rates of Stem Cell Therapy for Autism
Research and clinical experience increasingly demonstrate that an increasing number of children with autism show improvement after stem cell therapy. For example, an Indian study of 32 children reported that 91% improved on a standard autism scale, and 62% showed reduced symptom severity.
Most parents who tried stem cell therapy for autistic children in India or other countries also noted global improvement. While these findings are encouraging, the absence of placebo groups necessitates careful interpretation of the results.
Reported improvements commonly include:
- Stronger eye contact and social engagement.
- Better focus, attention, and presence.
- Gains in speech and communication.
- Calmer behavior with fewer meltdowns.
- Reduced repetitive behaviors.
- Improved sleep and digestion.
- Faster learning in therapy or school.
Swiss Medica also achieves a high success rate in stem cell therapy for autism: nearly 80% of families observe significant benefits within the first few months.
“We decided to try stem cell therapy for our 9-year-old son with autism, and the results have been truly encouraging. Within days, we noticed changes we had never seen before—he confidently walked up to another child, shook hands, and said hello for the first time in his life.
The next morning, he brushed his teeth completely on his own without reminders, something we had always struggled with. These may seem like small steps, but for us they were huge milestones.”
Jogesh, United States
Types of Cells Used in Stem Cell Therapy for Autism in India
Different clinics may use different sources of stem cells or slightly different cell types. In case of stem cell therapy for autistic children In India, programs predominantly use MSCs, which we introduced earlier. MSCs are preferred due to their multipotency (capability to repair diverse tissues), relative ease of acquisition, and essential immunomodulatory characteristics.
Mesenchymal Stem Cells
MSCs can be found in two common sources for treatment:
- Umbilical cord/placenta (allogeneic donor cells) MSCs collected after healthy births.
- Bone marrow autologous cells that are taken from the patient.
Other cell-based options for stem cell therapy for autism in India may include exosomes—tiny vesicles released by MSCs that deliver proteins, RNA, and lipids to support healing—and macrophages, immune cells that clear debris, reduce inflammation, and promote repair.
These therapies work together to calm inflammation, balance the immune system, and support neurons. This may help with brain connectivity, social skills, communication, focus, and overall health.
Clinics Offering Stem Cell Therapy for Autism in India
There are a few centers that offer stem cell therapy for autistic children in India, providing stem cell–based treatments for autism. Their protocols are very different. Some use donor cells, others use the child’s own bone marrow, and many add extra treatments like detox, ozone, or intensive rehabilitation.
To help families understand the landscape and avoid risks of stem cell therapy in India, we’ve created an overview of the main clinics and their approaches in the table below.
| Clinic / Location | Approach & Protocol Highlights | Notes for Families |
| Dr. Pravin Patel Innovative Hospital (Vadodara) | Offers stem cell therapy combined with alternative autism treatments such as ozone and “quantum” therapies. | Broad treatment claims across many conditions; marketed via medical tourism platforms. |
| StemRx Bioscience Solutions (Mumbai) | Uses cord-derived cells and combines them with detox programs, microbiota transfer, and adjunct therapies. | Promotes wide-ranging regenerative medicine; strong marketing language. |
| GIOSTAR Hospital (Bengaluru) | International network advertising advanced stem cell protocols for neurological and other diseases. | Known for broad claims (autism, diabetes, orthopedics); details on autism-specific protocols are limited. |
| NeuroGen Brain & Spine Institute (Mumbai) | Uses the child’s own bone marrow cells, administered with intensive rehabilitation (rule of 6: six hours/day, six days/week, six months). | Emphasizes rehab alongside cell therapy; highlights large patient numbers. |
The above centers have various track records: some lean more academic, while others are more oriented to medical tourism.
This is not to say that you should go to any specific clinic; instead, you should conduct your own research, read reviews, speak with previous patients, and seek advice from doctors who are not affiliated with the clinic.
Cost of Stem Cell Therapy for Autism in India
The cost of stem cell treatment for autism in India typically ranges between USD $18,000–$40,000 for one treatment cycle. This is less than in the United States or Europe, where similar programs frequently cost $20,000-$50,000 or more, but still more than in Serbia or Panama.
Why is there such a big difference in stem cell therapy costs? The final price of autism treatments in India and other countries is based on several factors:
- Cell source and lab work (donor cord cells vs. child’s own bone marrow).
- Extra program costs (rehabilitation therapies, length of stay, housing, transportation, and support services).
- Taxes and operating costs differ from country to country, depending on the cost of living and economic rules in each place.
Cost of Stem Cell Therapy for Autism in India vs. International Options
To put it simply, the cheapest countries for stem cell treatment are Serbia, Panama, and India. Here is a summary of costs for stem cells in India vs Europe and the USA.
| Location | Typical Cost (USD) |
| India | $18,000–$40,000 |
| Serbia | €7,000 – €31,000 (at Swiss Medica €7,000 – €19,000)* |
| Panama | $15,000–$18,000 |
| USA | $20,000–$50,000 |
*Prices are indicative, based on January 2025, and may vary with condition severity and cell quantity required.
The cost of stem cell therapy for autism in India and other countries naturally matters to every family, but it shouldn’t be the only focus. What truly counts is that the clinic provides safe, high-quality care for your child. The rules, standards, and oversight of medicine can be different from one country to the next, which can affect prices.
Next, we’ll look at how India regulates stem cell therapies and compare this to how other countries do it.
Regulation, Ethics, and Challenges of Stem Cell Therapy in India
The regulatory framework for stem cell therapy in India is still evolving. This creates ethical concerns and practical challenges for families who may encounter clinics offering treatments that fall into a regulatory gray area.
While India has strong rules on paper, enforcement and oversight remain inconsistent, leaving families to navigate uncertainty when considering these therapies.
Current Indian Guidelines
That is critical to understand for families looking for stem cell treatments for autistic children in India: the country has taken a cautious approach to stem cell treatments for unapproved uses, at least on paper. In 2019, the government’s New Drugs and Clinical Trial (NDCT) Rules explicitly defined any “cell or stem cell-derived product” (except minimally manipulated autologous cells for homologous use) as a “new drug.” This means that offering stem cell therapy for conditions like autism is supposed to happen only as part of approved clinical trials with the oversight of the Central Drugs Standard Control Organization (CDSCO).
The national body (NAC-SCRT) was disbanded in 2024, and approvals are now handled by each hospital’s ethics committee, which includes stem cell experts. This expedites the process but eliminates a central registry and clear national standards.
Updated guidelines (2025)
By 2025, expert groups in neurology, pediatrics, and other fields stressed that stem cell therapy should not be routine outside clinical trials. For autism, leading specialists and the Indian Academy of Pediatrics continue to call it experimental and recommend focusing on proven therapies first.
Current ambiguity
With no national oversight body, the rules are harder to enforce. In practice, some clinics still offer autism stem cell therapy, calling it “innovative” or a type of clinical study. Larger hospitals may review cases carefully, but smaller private clinics can sometimes bypass strict checks.
Ethical and Practical Challenges Families Face
Here are the main concerns patients in India usually consider before facing a treatment:
| Challenge | What It Means for Families |
| Regulatory gray zones | Some clinics still offer autism stem cell therapy as “innovative” or under trial labels, despite official bans. Enforcement is inconsistent, leading to confusion about what is actually allowed. |
| Variable standards | No unified protocol exists. Clinics differ in cell type, dose, and methods (IV vs. intrathecal, with/without therapies). Quality can vary greatly between large hospitals and smaller clinics. |
| Limited follow-up care | Many clinics focus on delivering treatment but provide little structured aftercare. Families may be left to manage therapies and concerns at home, with uneven support. |
| Enforcement gaps | India has strong laws on paper, but oversight has been inconsistent. Clinics may operate openly until a crackdown, making the system less predictable than in countries with tighter control (e.g., UAE and Serbia stem cell clinics). |
These challenges lead many families in India to explore global autism treatment options.
Alternatives Outside India
Stem cell therapy is available in several countries around the world, each with its own regulatory environment. Let’s take a look at some of the most popular destinations, along with their benefits and drawbacks.
Which Country Is Best for Autism Stem Cell Therapy?
There is no single “best” country or “best” therapy for autism. However, regulations differ—some countries have stricter oversight, while others are more flexible. Families should consider safety, access, cost, and regulation when making their decision.
Below is a comparison of popular destinations of stem cell therapy in Europe and other regions.
| Country | Pros | Cons |
| United States (clinical trials only) | Rigorous FDA oversight, world-class autism stem cell research. Highest bar for consent, safety monitoring, and data collection. | Only available via trials, strict criteria, limited spots, possible placebo groups. No commercial access. |
| European Union | Strong Advanced Therapy Medicinal Products (ATMPs) framework, hospital exemptions possible in some countries. | Very limited access, often case-by-case in academic hospitals. Heavy paperwork, rarely open to medical tourists. |
| Serbia | Legal framework aligned with the EU; governmental authorization required, resulting in fewer but more controlled clinics. More affordable autism treatment abroad. | Small market, limited providers. |
| Panama | Panama’s Stem Cell Institute published transparent protocols and costs for therapies with umbilical cords MSCs. | Not FDA/EMA approved. Oversight is internal, evidence still limited, families must scrutinize informed consent and follow-up. |
| Mexico | Convenient for North America, lower costs, some licensed labs and trials. COFEPRIS is tightening rules. | Quality highly variable. History of grey-zone clinics, aggressive marketing, wide price ranges. Must carefully verify credentials. |
Why Families Choose Swiss Medica in Europe
Some European clinics offer a balance of regulated access, transparent protocols, and comprehensive care, making them appealing to families. Swiss Medica, which is situated in Belgrade, Serbia, is one of them.
Swiss Medica in Serbia: A Safer Path
- Clear regulation: In Serbia, clinics can only provide stem cell therapies with authorization from the Ministry of Health, which ensures oversight and reduces the risk of unregulated practice. Swiss Medica holds this authorization.
- European quality standards: Treatments use GMP-certified labs, board-certified doctors, and ethics committee reviews. Protocols align with European expectations for safety and professionalism.
- Accessible location: Belgrade is centrally located in Europe, with visa-free entrance for many countries. Patients with Indian passports who hold valid Schengen, UK, or US visas or residence permits may enter Serbia for up to 90 days without a Serbian visa.
- Comprehensive care: Programs at Swiss Medica include not just stem cell infusions but also rehabilitation, nutritional support, and structured follow-up, offering a holistic approach.
- Transparency: Swiss Medica shares details on cell sources, dosage, and methods, emphasizing realistic outcomes for patients.
What Makes Swiss Medica Different
Swiss Medica has been treating ASD conditions since 2011. During this period, we established our own hospital, lab, and team of professional regenerative specialists.
- Experience: We have over 14 years of regenerative therapy experience, 1000+ children with ASD who have made significant improvements, and patients from all over the world.
- Safety: We produce specialized MSC-based products in a laboratory that strictly follows GMP standards.
- Personalized care: Our multidisciplinary team ensures a low doctor-to-patient ratio (one doctor for every 3–4 patients).
- Not just injections: For ASD patients, we have developed comprehensive programs that include thorough evaluation, additional procedures such as speech and physical therapy, and ongoing follow-up care. We do not simply discharge patients after the main treatment—our doctors remain in contact and monitor progress for 3–6 months.
- Individualized protocols: We provide customized dosing and delivery (IV, intrathecal, local) plus rehabilitation and supportive therapies.
- Transparency: We want every family to make well-informed decisions before starting therapy. That’s why our doctors and medical advisors stay in close contact, providing a clear understanding of each stage of the child’s treatment.
Swiss Medica’s hospital is a large medical center with its own lab and family-friendly accommodations.
Testimonials and Real Results
Here are a few real testimonials that show the kinds of improvements parents have seen after their children had stem cell therapy at Swiss Medica:
“The hardest part for us as parents was that Luca was nonverbal—he couldn’t communicate his needs, which led to frustration and sleep difficulties. After arriving at Swiss Medica, we were nervous, but the care and support we received exceeded our expectations. The staff were warm, compassionate, and made us feel safe from the very beginning.
Luca adapted far better than we imagined—he was calm, comfortable, and responded really well to the treatment. For us, even hearing him start to say a few words feels like a huge step forward. More than anything, we now have hope that he will continue to develop his ability to communicate. This experience has been truly positive and uplifting for our whole family.”
— Joseph, father of Luca, Australia
“Before treatment, my son couldn’t speak, barely slept, and suffered constant seizures. Just days after stem cell therapy, he surprised us by saying his first full sentence—something we never thought possible. Now he communicates, sleeps through the night, and lives a calmer, happier life.”
— Sheila Breiner, mother and nurse
Next Steps for Families Exploring Stem Cell Therapy
Choosing stem cell therapy for an autistic child in India or other countries is a major step that comes with many hopes and questions. Here, we outline how families can prepare for the decision about Swiss Medica autism therapy and what follows after treatment.
Step 1. Book a Professional Consultation
Your journey starts with a free online consultation with a Swiss Medica doctor. During this call, you can talk about your child’s past and ask any questions you have, such as what kinds of stem cells are used and what improvements might be possible. This stage is all about exploring your options in a supportive, no-pressure environment.
Step 2. Undergo a Thorough Medical Evaluation
If you choose to proceed, we will schedule a comprehensive medical evaluation for your child. The focus is always on safety and relevance to your child’s condition. We collect medical history, review laboratory tests (including bloodwork and hormone panels), and conduct evaluations by a neurologist and pediatrician.
We also check on neurological and psychological health, as well as speech and behavior growth. If your child has co-existing conditions, further diagnostic tests may be recommended.
Step 3. Plan Realistically for the Journey
Don’t expect results right away or miracles. Instead, set realistic goals—such as improved eye contact, clearer communication, or more stable sleep. Continue existing therapies like speech, ABA, and occupational therapy, as they are essential to reinforcing and building upon any progress achieved.
Most importantly, be patient. Real change often unfolds month by month, not overnight. Support your child’s health with good nutrition, hydration, rest, and a peaceful routine.
Contact us
Take the first step for your child—book a free online consultation with our doctors. No pressure, no commitment, just guidance for your child’s health.

Medical Advisor, Swiss Medica doctor
Frequently Asked Questions
1. Is stem cell therapy a cure for autism?
No, it is not a cure; it may help reduce symptoms and support progress, but autism remains a lifelong condition.
2. How soon will we see changes after therapy?
Some children show improvements within weeks, while others may take 3–6 months, and outcomes vary by child.
3. Is stem cell therapy for autism legal in India?
Stem cell therapy for autism is not officially approved in India. According to Indian regulations, such treatments should only be offered within registered clinical trials. However, because oversight is now decentralized to hospital ethics committees, some private clinics continue to provide stem cell therapies for autistic children in India.
4. What is the cost of stem cell therapy for autism in India and other countries, and is it covered by insurance?
Stem cell therapy costs in India and other countries range from $7,000–$50,000, and insurance usually does not cover it.
List of References:
Uke P, Gaikwad S, Vagha K, Wandile S. Unraveling the Spectrum: A Comprehensive Review of Autism Spectrum Disorder in India. Cureus. 2024 Jun 20;16(6):e62753. doi.org/10.7759/cureus.62753
Ping Tu, Xirongguli Halili, Siyi Zhang, Jing Yang, Yongbei Xiao, The effectiveness of hyperbaric oxygen therapy in children and adolescents and with autism spectrum disorders: A systematic review and meta-analysis, Progress in Neuro-Psychopharmacology and Biological Psychiatry, Volume 137, 2025, https://doi.org/10.1016/j.pnpbp.2025.111257
Rauter Alexandra , Schneider Horst , Prinz Wolfgang. Effectivity of ILF Neurofeedback on Autism Spectrum Disorder—A Case Study, Frontiers in Human Neuroscience, Volume 16 – 2022. doi.org/10.3389/fnhum.2022.892296
Wong, R.S.Y. Neuroinflammation in autism spectrum disorders: potential target for mesenchymal stem cell-based therapy. Egypt J Neurol Psychiatry Neurosurg 58, 91 (2022). https://doi.org/10.1186/s41983-022-00525-2
Wang, Y., Yi, H. & Song, Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther 12, 545 (2021). https://doi.org/10.1186/s13287-021-02609-x
Akat, A., Karaöz, E. Cell therapies for autism spectrum disorder: a systematic review of clinical applications. Middle East Curr Psychiatry 30, 94 (2023). https://doi.org/10.1186/s43045-023-00363-9
Sharma A, Gokulchandran N, Sane H, Nagrajan A, Paranjape A, Kulkarni P, Shetty A, Mishra P, Kali M, Biju H, Badhe P. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem Cells Int. 2013;2013:623875. doi.org/10.1155/2013/623875
MD, Pediatrician, Regenerative Medicine Specialist











