Stem cell treatments in the USA have evolved from exploratory interest to a helpful alternative discussed in many specialist practices. As the evidence base grows and regulatory pathways mature, patients now have more opportunities—and more questions—than ever before about where to find safe, effective care. This overview explains how US stem cell companies operate today, what the benefits are, and why maintaining an informed, collaborative relationship with a qualified provider remains essential.
What Are Stem Cell Treatments?
Stem cells are the body’s master cells. When used in therapy, they can repair tissue that has been damaged by disease, trauma, or simple wear and tear. These treatments involve harvesting a patient’s own (autologous) or donor (allogeneic) stem cells, processing them under controlled conditions, and delivering them to injured or diseased tissue to stimulate repair or modulate immunity.
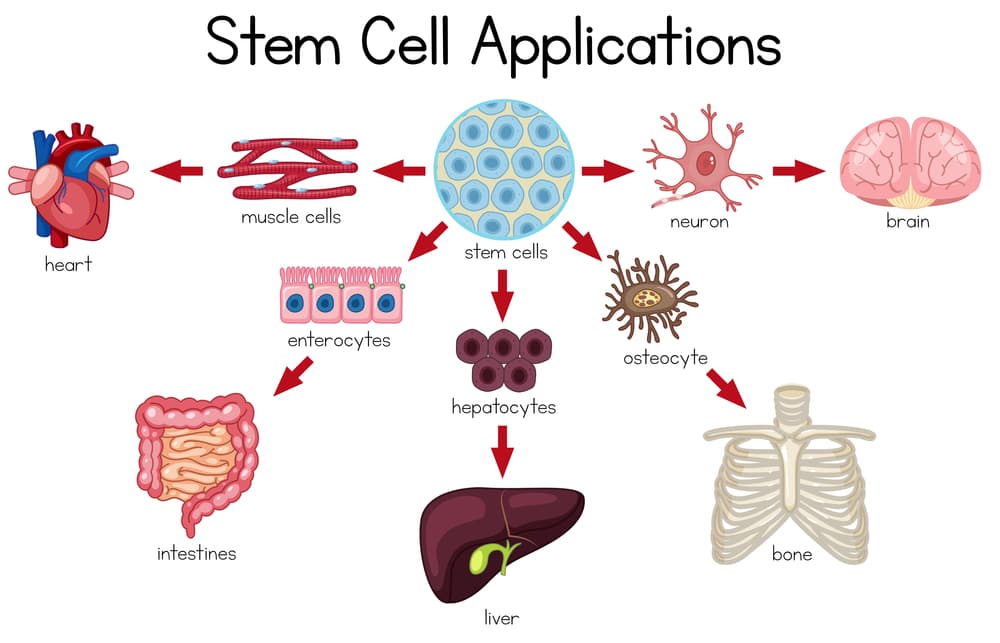
Adult multipotent mesenchymal stem cells (MSCs) are commonly utilized in the US and around the world for the treatment of degenerative diseases and certain diseases of the skin, bone, and cartilage.
There are several categories:
| Stem cell type | Source | Therapeutic effect |
| Hematopoietic stem cells (HSCs) | Bone marrow, mobilized peripheral blood, and umbilical cord tissue. | Reconstitute the blood and immune systems. |
| Mesenchymal stem cells (MSCs) | Bone marrow, adipose tissue, umbilical cord tissue. | Provide potent immunomodulation and promote tissue repair by activating the patient’s own regenerative capacity. |
| Induced pluripotent stem cells (iPSCs) | Adult somatic cells reprogrammed in vitro. | Used for customized disease modeling and gene-edited cell or tissue replacement. |
| Embryonic stem cells (ESCs) | Inner cell mass of the embryos donated after in vitro fertilization. Embryonic stem cells may pose a risk of uncontrolled growth or tumor formation. | Possess true pluripotency—capable of differentiating into virtually any cell type; show promise for retinal, spinal cord, and pancreatic islet repair. However, they may carry a risk of uncontrolled growth or tumor formation. |
How Stem Cell Treatments in the USA Are Regulated
The use of stem cells is regulated by the U.S. Food and Drug Administration (FDA), although not all stem cell treatments have been scientifically proven to be effective.
Role of the FDA
The FDA controls all medication products. Any therapy that claims to treat, cure, or mitigate disease must pass the same Investigational New Drug (IND) pathway required of pharmaceuticals. The agency also inspects manufacturing labs under its Current Good Manufacturing Practice (cGMP) rules.
Approved vs. Unapproved Treatments
Only a handful of stem cell therapies in the USA have full FDA approval, primarily for hematologic cancers that require stem cell transplants in USA hospitals. Most other procedures can be offered only within strict guidelines. Patients are advised to verify whether the protocol is part of an FDA-cleared trial, an FDA-licensed product, or a procedure operating under enforcement discretion.
Which Conditions Are Commonly Treated with Stem Cells in the US?
The FDA has approved the use of stem cell treatments in the USA, mainly for the treatment of blood and immune system diseases. Therapies for recovery from spinal cord injury, Parkinson’s disease and other conditions are being experimentally investigated, but these methods are not yet widely accepted.
Neurology
- Multiple sclerosis (MS);
- Parkinson’s disease;
- Spinal cord injury;
- Autism and autism spectrum disorders (ASD): The application of stem cell therapy for autism reflects increasing interest in neurodevelopmental interventions, although evidence remains limited.
Orthopedics
Autoimmune & Inflammatory Disorders
- Crohn’s disease;
- Systemic lupus erythematosus (SLE);
- Rheumatoid arthritis (RA).
Sports Injuries
- Partial rotator‑cuff tears and Achilles tendinopathy.
Get a free online consultation
You can contact our medical advisor to discuss your health condition with a specialist in regenerative medicine. It is free and confidential.

Medical Advisor, Swiss Medica doctor
The Process of the Stem Cell Treatment in the USA
Consultation & Evaluation
First, the patient undergoes an initial consultation and comprehensive evaluation at stem cell therapy centers in the USA. The medical team reviews the patient’s medical history, analyzes diagnostic imaging (MRI, X-rays) and conducts laboratory tests. This phase includes a transparent discussion of evidence-based outcomes, realistic expectations, and alternative treatment options.
Collection & Preparation
For autologous transplants, cells are typically obtained by bone marrow aspiration (from the iliac crest) or liposuction (for adipose-derived stem cells). The cells are then either concentrated for immediate point-of-care use or processed in a cGMP-certified laboratory for expansion and quality control.
Administration & Follow-up
The final phase involves precise delivery and long-term monitoring. Stem cells are administered via image-guided injection (using ultrasound or fluoroscopy for joint/spine treatments) or intravenous infusion for systemic conditions. After the stem cell transplant in the USA, patients follow a scheduled follow-up protocol with evaluations at 1 week, 1 month, 3 months and annually to track progress and therapeutic efficacy.
Benefits of Stem Cell Therapy for Patients in the USA
As regenerative medicine advances, stem cell therapy is emerging as a promising addition to conventional treatments, offering minimally invasive solutions with the potential for long-term healing. For patients seeking the best stem cell treatment in the USA, this advanced approach provides key characteristics over traditional methods, from reduced recovery times to addressing the root cause of disease rather than just managing symptoms.
| Advantages of stem cells | Traditional treatment |
| The procedure is typically minimally invasive, involving injections or small incisions. | Often invasive, up to and including such things as joint replacements or organ surgeries. |
| In most cases, the therapy uses autologous cells derived from the patient’s own body. | It often involves pharmaceutical agents, donor tissues, or synthetic implants. |
| Stem cell treatment may reduce the need for long-term use of steroids or immunosuppressants. | Typically, it requires lifelong use of medications such as painkillers. |
| In addition to alleviating symptoms, stem cell therapy has the potential to regenerate damaged tissue. | Generally focuses on symptom management without promoting tissue repair. |
| Recovery often takes just a few days to a few weeks in orthopedic applications. | Recovery may take weeks to months following major surgical interventions. |
| It may involve higher initial costs but reduce long-term healthcare expenditures. | Entails recurring expenses related to medications, revisions, and complications. |
It is important to realize that the combination of traditional therapies with stem cell therapies gives the best results in managing chronic conditions.
Risks and Controversies Surrounding Stem Cell Treatments
Stem cell therapy in the United States is still an innovative off-label approach. Therefore, it is essential to choose your treatment clinic carefully in order to avoid the following risks:
- Limited proof: Outside hematology, long-term randomized data remain scarce.
- Cost vs. benefit: High prices can lure patients into medical debt without guaranteed results.
- Tumorigenicity: Embryonic or poorly characterized cells can form unwanted growths.
- Ethical questions: Embryonic sources raise moral debates about the onset of human life.
Costs of Stem Cell Treatments in the USA
Stem cell therapy in the US is not covered by insurance, so it is important to evaluate the cost of the full treatment.
| Component | Typical range (USD) |
| Initial consult + imaging | $800–$2,500 |
| Cell harvest | $5,000–$12,000 |
| Stem cell administration | $3,500–$9,000 per site |
| Long-term follow-up package | $600–$1,500 |
If a center for stem cell therapy in the USA cannot itemize costs transparently, it should be considered a red flag.
For many American patients, the high cost of stem cell treatment in the USA can be a real obstacle. To make therapy more accessible, some people look abroad for high-quality care that’s also more affordable.
One such destination is Swiss Medica in Belgrade, Serbia, a popular choice thanks to its transparent pricing and proven results. The full cost of treatment at our stem cell clinic ranges from €7,000 to €31,000*, including accommodation.
*The prices mentioned are indicative and subject to change based on individual factors, including the condition’s severity and the number of stem cells needed. Prices are valid as of January 2025.
Contact us
Want to estimate the cost of stem cell treatment at Swiss Medica for your condition?
Contact us today for a free, no-obligation consultation.

Medical Advisor, Swiss Medica doctor
Current Research and Breakthroughs in the USA
Recent stem cell research in the US has achieved promising results by combining various stem cell types (embryonic, progenitor, iPSC) with gene editing tools (CRISPR-Cas9, TALENs) to develop genetically engineered stem cells.
These cutting-edge approaches, which underpin some of the best stem cell treatments in the USA, aim to treat complex neurological diseases by correcting genetic defects and enhancing therapeutic potential, opening new avenues for regenerative neurology.
The Future of Stem Cell Therapy in the USA
The next stage of regenerative medicine is taking shape, driven by cutting-edge innovations. Industry experts anticipate a new era of personalized care. As clinical evidence grows, expanded insurance coverage is expected to follow, making these therapies more accessible once their long-term cost-effectiveness is firmly established.
AI-powered imaging already helps predict which lesions are most likely to respond to treatment, guiding the development of the best stem cell therapy protocols tailored to each patient.
Should You Go Abroad? — A Nuanced Perspective
Stem cell therapy centers in the USA are not the only ones to offer cutting-edge regenerative treatments. International clinics, particularly in Europe and Asia, are also advancing rapidly, often providing innovative approaches, competitive pricing, and personalized care. Thorough research is essential before pursuing treatment abroad, as several critical factors must be considered, such as
- The destination’s regulatory standards
- The logistics of follow-up care
- Risks associated with travel, such as flight-related thrombosis
- The transparency of the cell processing laboratories.

Swiss Medica, based in Serbia, provides a personalized and effective approach to stem cell therapy, treating a wide range of conditions within a dedicated hospital setting. Our in-house laboratory ensures the highest standards of quality and safety. We exclusively use adult stem cells, which are free from the ethical concerns, tumorigenic potential, and unlimited division capacity associated with embryonic stem cells.
Frequently Asked Questions
1. How do I know I am receiving the best stem cell treatment in USA facilities?
Look for board-certified specialists, published outcome data, and in-house laboratories. Always verify the clinic’s registration number.
2. What is the best stem cell clinic in the US?
There is no universally superior provider; suitability depends on individual factors. Start with an accredited US stem cell companies for hematologic diseases or CTSA-backed academic hospitals for musculoskeletal trials. Evaluate based on investigator publication record, protocol transparency, and registry participation.
3. Are stem cells in the US derived only from embryos?
No. Embryonic sources are confined to tightly regulated laboratory research. Clinical treatments overwhelmingly use adult autologous or birth-tissue-donor-derived cells.
4. Will my insurance pay for stem cell therapy in the USA?
Commercial plans consistently cover FDA-approved bone marrow stem cell transplants in the USA for blood cancers and certain rare anemias. Elective orthopedic, neurologic, and autoimmune applications are still self-pay.
Contact us
Get a free online consultation to learn about the expected results of stem cell therapy in your case, along with details about treatment cost and duration.

Medical Advisor, Swiss Medica doctor
List of References:
Huang, L., Fu, C., Xiong, F., He, C., & Wei, Q. (2021). Stem Cell Therapy for Spinal Cord Injury. Cell transplantation, 30, 963689721989266. https://doi.org/10.1177/0963689721989266
Hoang, D. M., Pham, P. T., Bach, T. Q., Ngo, A. T. L., Nguyen, Q. T., Phan, T. T. K., Nguyen, G. H., Le, P. T. T., Hoang, V. T., Forsyth, N. R., Heke, M., & Nguyen, L. T. (2022). Stem cell-based therapy for human diseases. Signal transduction and targeted therapy, 7(1), 272. https://doi.org/10.1038/s41392-022-01134-4
Feng, J., Jang, G., Esteva, E., Adams, N. M., Jin, H., & Reizis, B. (2024). Clonal barcoding of endogenous adult hematopoietic stem cells reveals a spectrum of lineage contributions. Proceedings of the National Academy of Sciences of the United States of America, 121(4), e2317929121. https://doi.org/10.1073/pnas.2317929121
Chen KS, Koubek EJ, Sakowski SA, Feldman EL. Stem cell therapeutics and gene therapy for neurologic disorders. Neurotherapeutics. 2024 Jul;21(4):e00427. doi: 10.1016/j.neurot.2024.e00427
Turner L. The American stem cell sell in 2021: U.S. businesses selling unlicensed and unproven stem cell interventions. Cell Stem Cell. 2021 Nov 4;28(11):1891-1895. doi: 10.1016/j.stem.2021.10.008. PMID: 34739831.
MD, Pediatrician, Regenerative Medicine Specialist







